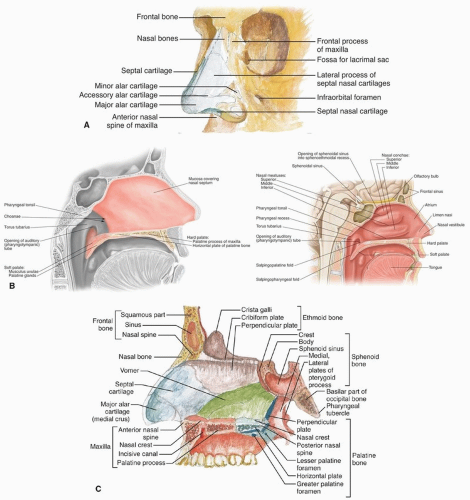
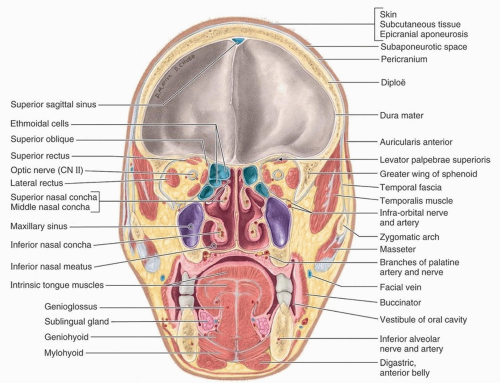
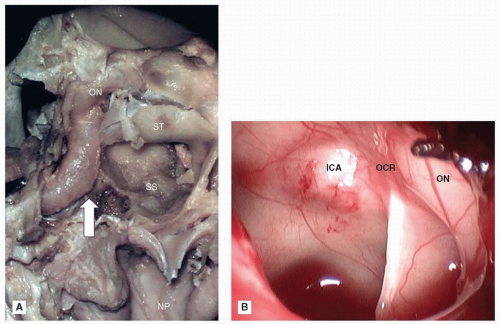
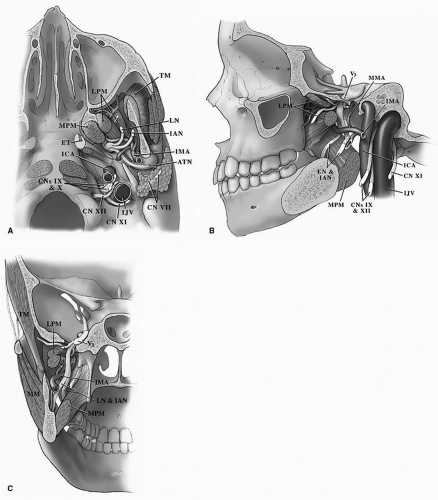
perpendicular plate of the ethmoid, its inferior margin with the vomer and the palatine process of the maxilla.
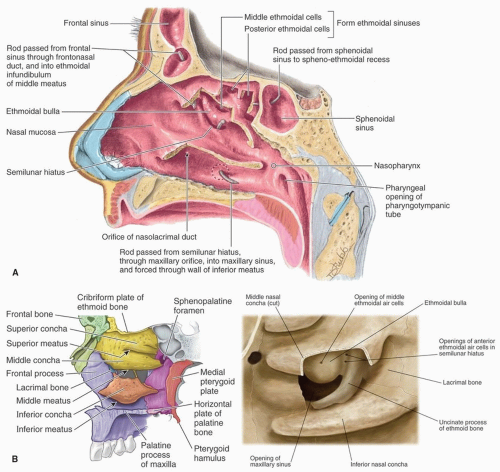
into a curved channel, the infundibulum, bounded above by the bulla ethmoidalis and below by the lateral surface of the uncinate process of the ethmoid. The anterior ethmoid air cells open into the front part of the infundibulum. The frontal sinus drains through the nasofrontal duct, which in ˜50% of subjects will also drain into the infundibulum; but when the anterior end of the uncinate process fuses with the front part of the bulla, this continuity is interrupted and the frontonasal duct then opens directly into the anterior end of the middle meatus. Below the bulla ethmoidalis, and partly hidden by the inferior end of the uncinate process, is the ostium of the maxillary sinus. An accessory ostium from the maxillary sinus is frequently present below the posterior end of the middle nasal concha. The inferior meatus is below and lateral to the inferior nasal turbinate. The nasolacrimal duct opens into the inferior meatus under cover of the anterior part of the inferior turbinate.
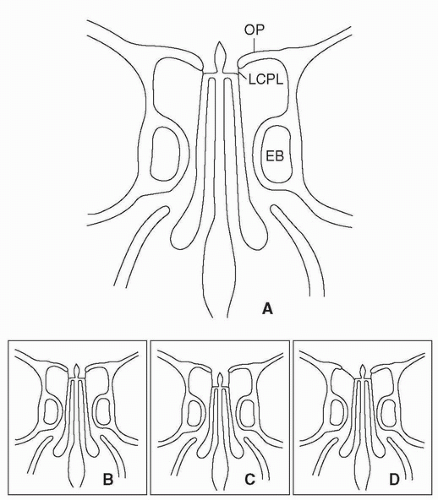
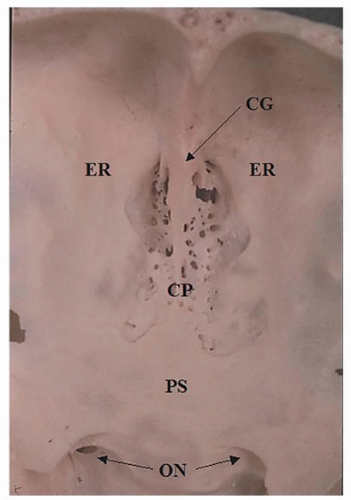
nasal roof, especially the relationship of the cribriform plate to the fovea ethmoidalis, is critical in avoiding a cerebrospinal fluid (CSF) leak during surgery in this region. The cribriform plate is usually at a slightly lower horizontal plane than the fovea ethmoidalis forming a shallow olfactory groove. This configuration is known as Keros type I (Fig. 10.4). However, the cribriform plate may be moderately or significantly lower than the fovea ethmoidalis resulting in a medium (Keros type II) or deep (Keros type III) olfactory groove. The topography of the roof may also be asymmetrical (Fig. 10.4).
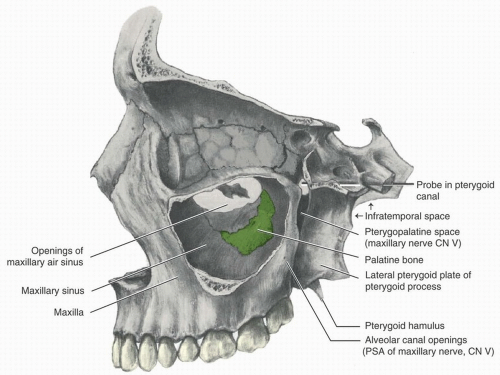 Figure 10.5. Anatomy of the maxillary sinus (lateral wall removed). (From Scheid RC, Weiss G, eds. Woelfel’s Dental Anatomy. 8th ed. Philadelphia, PA: Wolters Kluwer Health; 2012.) |
septum, which originates at the arcus marginalis, fusing with the levator aponeurosis above and the capsulopalpebral fascia below. It is bounded by the ethmoid and sphenoid sinuses at its medial aspect, the frontal sinus superomedially, the cranial vault superiorly and posteriorly, the temporal fossa laterally, and the maxillary sinus inferiorly. Each orbital cavity has a roof, a floor, a medial and a lateral wall, a base, and an apex.
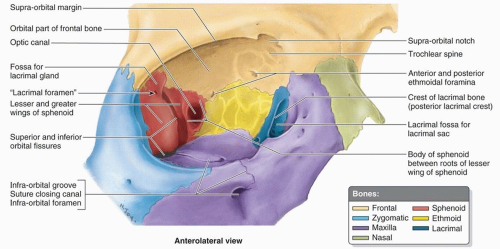 Figure 10.10. Anatomy of the right orbit. (From Moore KL, Agur AMR, Dalley AF, eds. Clinically Oriented Anatomy. 7th ed. Philadelphia, PA: Wolters Kluwer Health; 2013.) |
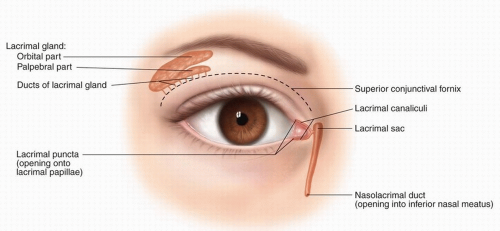 Figure 10.11. Anatomy of the lacrimal system. (From Pansky B, Gest TR, eds. Lippincott’s Concise Illustrated Anatomy: Head and Neck. Vol. 3. Philadelphia, PA: Wolters Kluwer Health; 2014.) |
the puncta, canaliculi, lacrimal sac, and nasolacrimal duct. Tumor involvement of the lacrimal system may present with epiphora.
region is reflected in the histogenesis of a complex variety of epithelial and nonepithelial tumors (Table 10.1). These tumors have a wide range of biologic behavior, and a few arise only in the SNT (e.g., inverted papilloma, olfactory neuroblastoma). Nonepithelial tumors are similar to those in other regions in the head and neck.
Table 10.1 Tumors of the Sinonasal Tract | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
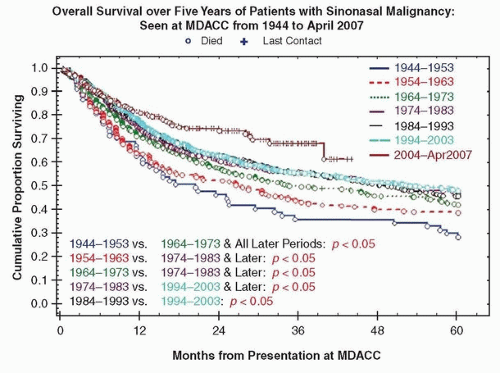
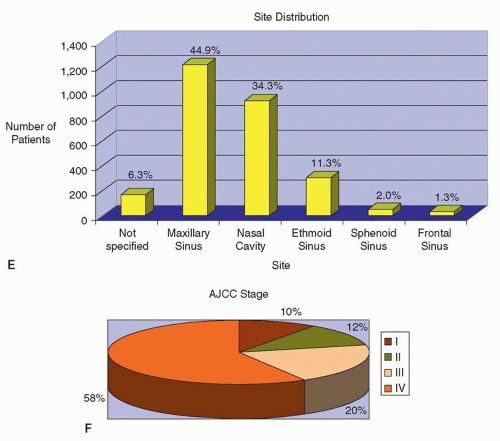
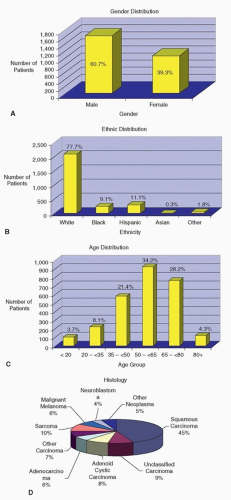 Figure 10.12. A-F: Patients with sinonasal cancer seen at MD Anderson Cancer Center between 1944 and April 2007 (n = 2,698 patients). |
Table 10.2 Classification of Sinonasal Cancer According to the AJCC 7th Edition | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
pterygoid plates, or tensor veli palatini muscle. Extension to the skull base may lead to involvement of the cranial nerves leading to anosmia, blurred vision, diplopia, or in hypoesthesia along the branches of the trigeminal nerves. The presence of associated neck masses usually represents metastatic disease in the cervical lymph nodes.
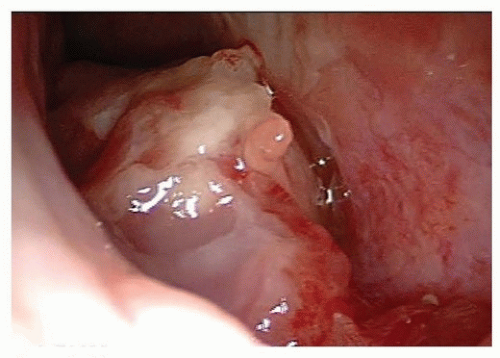 Figure 10.13. Carcinoma of the nasal cavity. Endoscopic view showing a tumor arising from the floor of the right nasal cavity. Biopsy revealed SCC. |
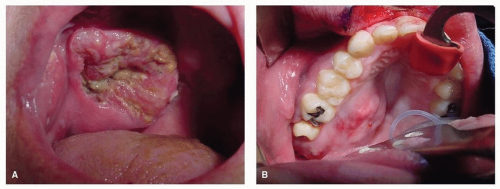 Figure 10.15. Carcinoma of the maxillary sinus may extend inferiorly and destroys the palate presenting as an ulcer (A) or submucosal mass (B). |
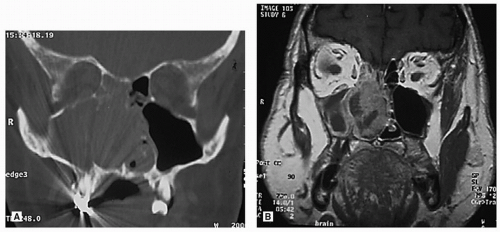
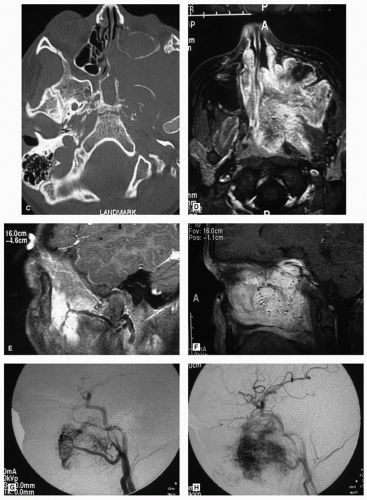
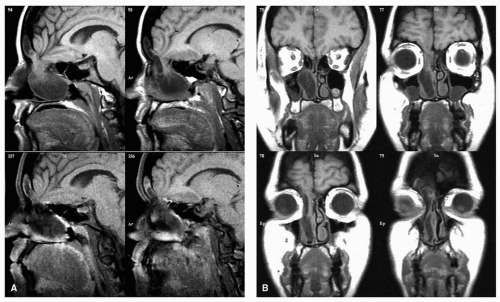
and bright illumination (Fig. 10.13). The application of topical anesthetics and decongestants improves visualization and allows thorough examination of the nasal cavity. The site of origin of the lesion and its relation to the nasal walls (septum, floor, roof, and lateral nasal wall) should be noted. An adequate specimen should be obtained, avoiding crushing of tissue, and submitted for histopathologic examination. If the diagnosis of lymphoma is suspected, fresh tissue should be sent in saline, rather than fixed in formalin. Most endonasal biopsies can be performed in the outpatient setting with minimal discomfort to the patient. In certain cases, the diagnosis of a highly vascular neoplasm, such as angiofibroma, may be suspected on clinical grounds. Under these circumstances, it is prudent not to perform the biopsy until imaging and angiography (possibly with embolization) are performed (Fig. 10.20). Preoperative biopsy can then be performed in the operating room under controlled conditions to confirm the diagnosis before surgical resection. If a nasal mass is suspected to have an intracranial communication such as an encephalocele, meningocele, or nasal glioma, this should be confirmed with imaging to avoid inadvertent CSF leak and subsequent meningitis (Fig. 10.21).
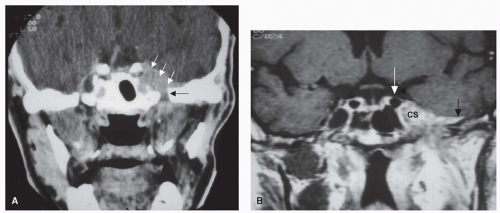
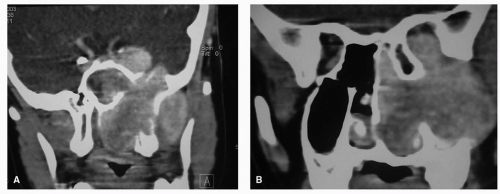
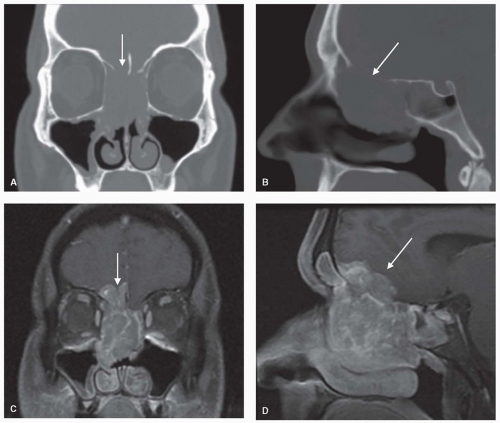 Figure 10.16. Ethmoid carcinoma. Coronal and sagittal images showing bony destruction on CT scan (A, B) and intracranial invasion (arrows) on T1-MRI with contrast (C, D). |
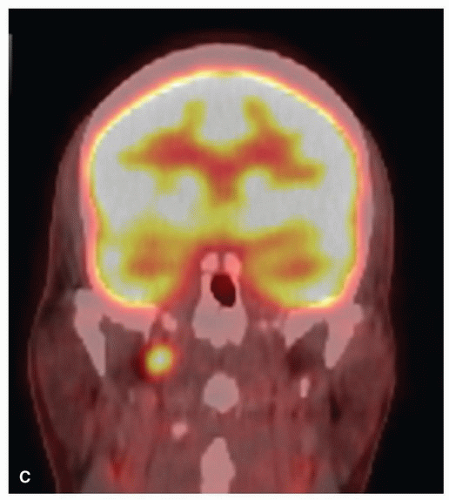
 Figure 10.19. PET-CT of the head and neck. These images are from the same patient whose CT and MRI are depicted in Figure 10.16. The fused PET-CT images show ethmoid carcinoma (A) with metastasis to the retropharyngeal lymph nodes (B, C), which were not detected on CT or MRI. |
involvement) or high-grade lesions (e.g., SNUC). In selected cases, chemotherapy and/or radiation may be reasonable alternatives to surgery. Such decisions are best discussed in the format of a multidisciplinary tumor board. If surgery is chosen as a treatment modality, the plan for the surgical approach, the extent of resection, and reconstructive options should then be formulated. This plan should be communicated clearly among the various members of the surgical team particularly the otolaryngologists, head and neck surgeons, neurosurgeons, and plastic and reconstructive surgeons.
Adequate oncologic resection
Minimal brain retraction
Protection of critical neurovascular structures
Table 10.3 Surgical Approaches and Extent of Resection of Cancers of the Sinonasal Tract
Surgical Approach
Endoscopic
Lateral rhinotomy and Weber-Fergusson
Transoral-transpalatal
Facial “degloving”
Craniofacial
Extent of Resection
Ethmoidectomy
Inferior maxillectomy
Medial maxillectomy
Total maxillectomy
Anterior cranial base resection
Infratemporal fossa dissection
Orbital exenteration
Meticulous reconstruction of the skull base
Optimal esthetic outcome
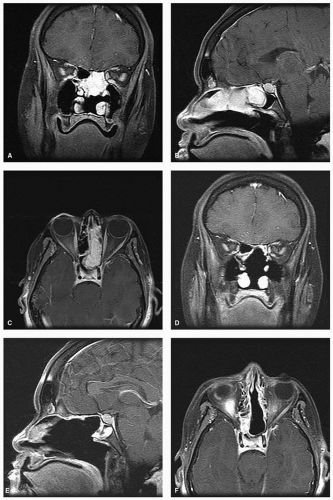
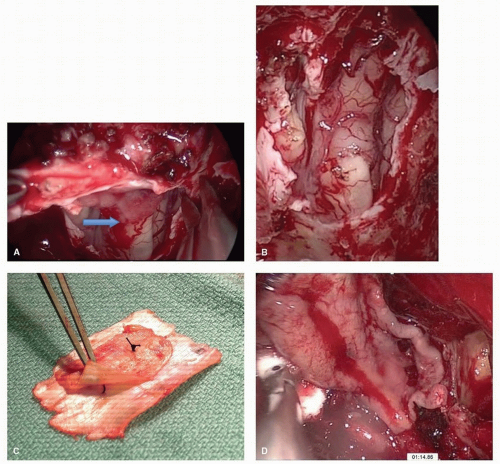
was associated with CSF leak rates of 20% to 30% for EEA of the anterior skull base.55,56 The application of a nasoseptal flap placed extradurally has lowered leak rates to 5% (Fig. 10.23).57 When there is tumor involvement of the superior nasal septum, the “extended nasoseptal flap” can be harvested from the lower septum and extended onto the floor and lateral wall of the nasal cavity.58 Other vascularized reconstructive alternatives for the anterior skull base include minimally invasive pericranial flap,59 the middle turbinate flap for small defects and the transpterygoid temporoparietal fascia flap.60,61 The inferior turbinate flap, while robust, has limited reach and is best suited to clival defects.62 Other flaps described in the literature such as the palatal flap,63 the buccinator myomucosal flap,64 and the occipital galeopericranial flap65 may be considered.
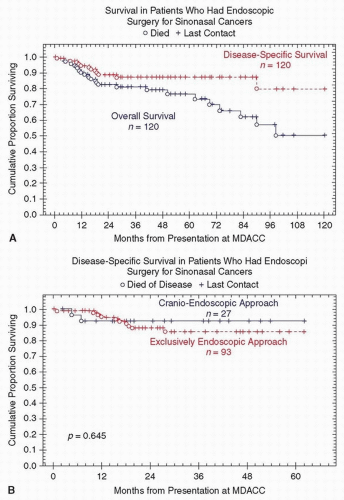
the role of appropriate adjuvant therapy and treatment by expert multidisciplinary teams in the management of sinonasal malignancies. Nicolai et al.68 reported on 184 patients from the University of Brescia and the University of Pavia/Insubria-Varese, treated from 1996 to 2006. The overall 5-year DSS was 82%. At the mean follow-up of 34 months, the local, regional, and distant recurrence was 15%, 1%, and 7% of patients, respectively. Both study cohorts had similar distributions of T staging, adjuvant treatment, and the proportion of EEA to CEA (Table 10.4). However, compared to the MDACC group, patients in the European group were older, were predominantly male, were less likely to have had prior treatment (28% vs. 58%), and were more likely to present with adenocarcinoma (37% vs. 14%). Irrespective of these differences, the 5-year DSS for the two series is comparable to those reported in the open anterior cranio-facial resection (ACFR) cohorts. Both groups concluded that in well-selected patients, endoscopic resection of sinonasal cancers results in acceptable oncologic outcomes.
Table 10.4 Comparison of Results from Two Large Studies of Oncologic Outcomes of Endoscopic Resection of Sinonasal Malignancy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree