39 Cancer of the Liver, Bile Duct, and Gallbladder
Anatomy
The liver is divided into two lobes demarcated by the middle hepatic vein. These lobes are further divided into eight segments based on the left and right hepatic veins and the portal vein. Fig. 39-1 demonstrates the Couinaud segments on frontal, posterior, and axial views of the liver. The liver receives a dual blood supply with approximately one-third coming from the portal vein and the remainder from the hepatic arteries. Venous drainage is via the hepatic veins, which empty into the inferior vena cava. The caudate lobe or segment 1 has a separate blood supply, receiving blood from both the right and left hepatic arteries and drainage directly to the inferior vena cava. Because of this division into self-contained units, each segment can be resected without damaging the remainder of the liver. However, there are no true anatomic borders between the segments; thus, tumors can often extend across liver segments.
Bile duct anatomy follows fairly predictable general patterns, with variants that may be important to the surgeon and occasionally of interest to the radiation oncologist. The liver ductules gradually coalesce into right and left hepatic ducts, which then form the common hepatic duct (known as the “upper” or proximal third). Fused with the cystic duct from the gallbladder, they form the common bile duct (the middle third), which, as it draws nearer to the ampulla of Vater, becomes known as the distal common bile duct (the lower or distal third) (Fig. 39-2). This is a lymph-rich system: lymphatic channels course throughout the submucosa of bile ducts and eventually drain into the porta hepatis, the pancreaticoduodenal nodes, and the celiac axis.
Primary Liver Tumors
Epidemiology and Etiology
In the United States, there were an estimated 22,620 new cases of primary liver cancer diagnosed and 18,160 deaths in 2009.1 The worldwide incidence of primary liver cancer is much more significant, with an estimated 1 million patients diagnosed annually.2 Hepatocellular carcinoma (HCC) represents approximately 70% to 85% of the cases. The incidence of HCC has been steadily increasing in the United States with a 75% increase during the past decade.3 The most common cause of HCC worldwide is hepatitis B, with other major causes including hepatitis C, alcoholism, and primary biliary cirrhosis. In addition, HCC has been linked with genetic hemochromatosis, hereditary tyrosinemia, α1-antitrypsin deficiency, aflatoxins, and possibly a promotional relation with alcohol. There is an estimated 0.5% annual risk of developing HCC in chronic hepatitis B patients and 5% in chronic hepatitis C patients.
Primary tumors of the bile ducts, also called cholangiocarcinomas, can be divided into intrahepatic (IHCC) or extrahepatic (EHCC) tumors (including hilar and distal bile duct tumors), and excludes tumors of the ampulla of Vater and gallbladder (see Fig. 39-2). HCCs and IHCCs are often grouped together as primary liver tumors, whereas EHCC and gallbladder tumors are treated as separate tumors of the biliary tree. The incidence and mortality from IHCC has been increasing during the last three decades.4 A host of predisposing factors have been proposed for bile duct tumors; the most widely accepted associations are with liver flukes, ulcerative colitis associated with primary sclerosing cholangitis, and congenital biliary cysts.
Pathologic Conditions
Histologically, early HCCs are generally well differentiated but can eventually show poor differentiation with marked pleomorphism. The only widely agreed-on HCC subcategory is the fibrolamellar type, which tends to occur in younger populations with less pre-existing liver disease and is associated with better outcome. The World Health Organization histologic classification5 recognizes several HCC variants, including childhood, spindle cell, clear cell, combined, carcinosarcoma, and sclerosis; these subcategories are used more, less, or not at all at varying institutions.
IHCCs are primarily adenocarcinomas, and are therefore difficult to distinguish from metastatic lesions from other primary sites involving the liver. There are no pathognomonic immunohistochemical stains distinguishing cholangiocarcinomas, although cytokeratin-7 positivity is often found. Special stains that help in the diagnosis to distinguish IHCC versus HCC include alpha-fetoprotein (AFP), which is positive in 35% to 75% of hepatomas and almost never in IHCC tumors; and CA19-9 or Ca-50, which is positive in 80% to 90% of cholangiocarcinomas and very rarely in hepatomas.6
Clinical Presentation
The presentation of primary liver tumors is often insidious, and clinical symptoms generally herald advanced disease. Patients often complain of antecedent malaise, anorexia, and occasionally abdominal pain. Signs include fever of unknown origin, night sweats, hepatomegaly, or a right-upper-quadrant mass, evidence of portal vein obstruction, bruits, ascites, splenomegaly, weight loss, or findings attributable to metastatic disease. In cirrhotic patients, in whom HCC is more common, unexplained weight loss can be a warning sign and some patients present in florid hepatic failure. Jaundice is less common in patients with primary liver tumors and may be associated with very advanced disease caused by biliary obstruction or hepatic failure resulting from extensive parenchymal destruction. Screening of high-risk patients with ultrasonography and measurement of AFP levels can help to detect small asymptomatic lesions, although the effect of early detection on survival is still unclear.7,8
Diagnostic Studies
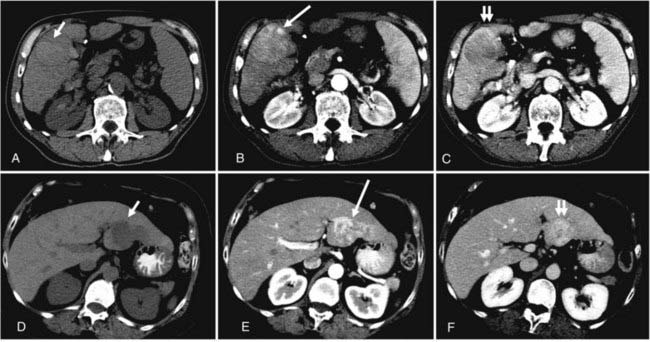
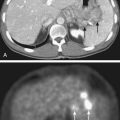
The list of imaging studies designed to evaluate local disease is headed by ultrasonography, which, by virtue of its low cost, sensitivity, noninvasive property, and relative simplicity of use, has been effective as both a screening and a diagnostic tool for primary liver tumors. It is useful in determining the number and size of lesions (lesions as small as 1 cm are detectable),7 ductal status, and vessel patency. Contrast-enhanced computed tomography (CT) scans have a somewhat higher sensitivity than ultrasonography (68% versus 60%) based on a pooled analysis of imaging studies for detecting HCC,9 with even higher sensitivity using triple-phase helical CT scans (arterial, portal venous, and delayed phases) of 89%.10 HCC tend to be hypervascular and are therefore best seen on the arterial phase, but appear hypodense or even isodense relative to liver parenchyma during the portal venous phase. IHCC tend to be hypodense during the portal venous phase and often hyperattenuated on delayed images. Peripheral contrast enhancement can also be seen in arterial or portal phase imaging.11 Fig. 39-3 shows an example of HCC and IHCC as seen on CT scan.
CT can also be valuable in evaluating disease beyond the liver and in both the detection of vessel invasion and the identification of vascular variants. Cirrhosis can seriously complicate the picture when evaluating the primary tumor on CT. Recent data suggest that contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive technique for detecting liver nodules, particularly in the setting of cirrhosis; dynamic hepatic arterial-phase contrast material–enhanced imaging is essential with both CT and MRI for visualization of small hepatic nodules in a background of cirrhosis.9,12–16 On MRI, IHCC can look very similar to metastatic colorectal cancer, but is often accompanied by intrahepatic biliary dilatation.17 Magnetic resonance cholangiopancreatography (MRCP) is a newer technique to noninvasively visualize the intrahepatic and extrahepatic bile ducts and the pancreatic duct to determine the extent of disease and potential resectability.
Positron emission tomography (PET) scans using fluorine-18–2-fluoro-2-deoxy-d-glucose (F-18 FDG) have not been routinely used for HCC because of the variable FDG avidity of these tumors; however, they appear to be of more benefit for IHCC, particularly for identifying extrahepatic metastatic lesions.18 Other metastatic studies for both HCC and IHCC include chest CT and bone scan. Of note, IHCC should be regarded as a diagnosis of exclusion because most adenocarcinomas involving the liver are from metastatic disease; thus a work-up to identify a primary site should be performed in the setting of a biopsy-proven adenocarcinoma involving the liver.
The benchmark laboratory study for detection and evaluation of treatment response is the serum AFP; it is elevated in 70% to 80% of all HCC patients. This rate is lower (≅30%) in the United States and Europe19 and in patients with fibrolamellar histologic findings. Overall, AFP, when used with a conventional cutoff point of 500 ng/mL, has a sensitivity of 50% and a specificity of 90% in detecting HCC in a patient with pre-existing liver disease.20 Higher levels are associated with poorer differentiation and shortened survival times.
Histologic confirmation of a hepatic tumor can be obtained through fine-needle aspiration, core biopsy, or open biopsy or resection. For centrally located IHCCs, tissue diagnosis may be a challenge. Endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiogram may be performed to obtain brushings for confirmation of a primary bile duct tumor. Some practitioners also obtain tissue from adjacent liver to determine the extent of coexistent hepatic disease. In patients with underlying liver disease, these procedures can be technically difficult to perform because of coagulopathies and tumor hypervascularity. In the absence of an elevated AFP, it is generally considered crucial to obtain a tissue diagnosis given the large differential and wide range of treatment options and prognoses. However, for tumors larger than 3 cm, with elevated AFP and clinical and radiographic findings suggesting HCC, the false-positive rate for diagnosis of HCC has been shown to be as low as 3%.21
Staging
Several staging systems have been proposed for HCC. Many clinicians categorize tumors as being (1) localized, (2) in liver only but in more than one lobe, or (3) metastatic. Okuda and colleagues13 incorporate tumor size (percentage of liver), ascites, and albumin (<3 g/dL) and bilirubin (>3 mg/dL) levels. The tumor-node-metastasis (TNM) staging system for both HCC and IHCC, as developed by the American Joint Committee on Cancer (AJCC), is presented in Table 39-1.
Therapeutic Modalities
Surgery
Surgery is currently the only potentially curative treatment modality for patients with primary liver tumors. In eligible patients, the surgical options are partial hepatectomy and liver transplantation. Less than 25% of HCC patients are technically and medically surgical candidates.22,23 Severe cirrhosis is often a contraindication to partial hepatectomy and can be best evaluated using Child-Pugh grade, which uses the severity of encephalopathy and ascites, as well as the absolute values for bilirubin, albumin, and PT; all Child C and many Child B patients are not candidates for surgery. Additional contraindications to resection include prolonged bleeding time, vascular invasion (particularly the portal or hepatic vein), and poor overall medical condition. Patients with IHCC are less likely to have underlying liver disease, but still are often unresectable because of other limitations to curative surgical excision, including lymphadenopathy, vascular involvement, metastases, and multilobar involvement.24
Modern series of hepatic resection for HCC report operative mortality rates of less than 5%,22 because of modern diagnostic, surgical, anesthetic, and postoperative monitoring techniques. Chen and associates24 had a 4% operative mortality in 120 patients in China, 46% of whom had cirrhosis (none were classified as Child C). Postoperative morbidity rates have also varied; most modern surveys show severe complication rates of 5% to 10%. These include bleeding, hepatic failure, subphrenic abscess, sepsis, pleural effusion, and bile leakage.24,25
Adverse prognostic factors for recurrence of HCC after liver resection include tumor size, elevated bilirubin or alkaline phosphatase levels, profound weight loss (>25%), ascites, extracapsular extension, positive margins, portal vein involvement, and nonfibrolamellar histologic findings.7,22,24 Ikeda and associates25 reported higher recurrence rates after curative resection if there were multiple nodules, high histologic grade, or negative hepatitis C antibody results.
Okuda and associates13 observed a 7-year overall survival rate of 45% in their select group of HCC patients who underwent resection with curative intent. Ikeda and associates25 reported a 5-year cumulative survival rate of 69% in patients managed very aggressively following recurrence after curative resection. Other surgical survival rates are lower: Chen and associates24 had a 26% 5-year rate, and rates in the United States are generally under 35%.26
Operative therapy for IHCC is often hampered by positive resection margins, even with extensive resection, often including removal of adjacent structures, such as the extrahepatic biliary tree, vascular structures, the diaphragm, or bowel.27 Carpizo and D’Angelica27 cite Lang et al., who evaluated the outcomes of 27 patients who underwent resection for IHCC and demonstrated that achieving an R0 resection was associated with a better survival. The addition of a portal lymph node dissection for IHCC is controversial and may be based on the level of suspicion of metastatic invovlement.27
Liver transplantation can be a curative option for patients with primary liver tumors who fulfill the selection criteria. In addition, transplantation restores liver function in patients with underlying cirrhosis and addresses the potential for multifocal disease.28 Ringe and Iwatsuki’s 1- and 3-year survival rates for liver transplantation were, respectively: stage I, 75% and 75%; stage II, 80% and 60%; stage III, 60% and 40%; and stage IVa, 50% and 15%.29,30 However, given the high-risk of liver transplantation and the limited organs available, strict selection criteria have been established to identify patients most likely to benefit from transplantation. Using the Milan criteria (solitary tumors of less than 5 cm and in those who have up to three tumor nodules, each of which is smaller than 3 cm), excellent results of transplantation for HCC have been reported, with 5-year survival rates exceeding 70%, a rate similar to that in patients who undergo liver transplantation for a nonmalignant disease.31
The role of liver transplantation for IHCC is less well defined than that for HCC. After early enthusiasm, studies of transplant for even the earliest stage IHCC have shown poor outcomes. However, investigators from the Mayo Clinic reported encouraging outcomes with neoadjuvant chemoradiation followed by liver transplantation.32 Eligibility included unresectable IHCC or IHCC in the setting of primary sclerosing cholangitis with no extrahepatic or lymph node metastases as determined by laparotomy. Of 71 patients enrolled, 38 underwent liver transplantation. The 3- and 5-year survival rates for this group were 82% and 82%, respectively. These results compared favorably to another group of patients that underwent resection whose 3- and 5-year survival rates were 48% and 21%, respectively.
Systemic Therapy
The use of chemotherapy for primary liver tumors has been disappointing. For HCC, intravenous agents, both single and multiple, have an overall response rate of 0% to 20% (average around 15%) with virtually all of these being partial responses. Intra-arterial chemotherapy has a higher response range (15% to 70%, average about 50%),22 but has not been proven to improve survival.33
Chemotherapy for IHCC also has a limited role. Historically, fluorouracil (5-FU)–based chemotherapy regimens have had poor response rates; however, with gemcitabine, response rates have improved. Several phase II studies have looked at the combination of gemcitabine and cisplatin for advanced cholangiocarcinomas, showing more promising results with response rates in the range of 17% to 35%.34–37 Regional chemotherapy via a hepatic artery infusion is also being investigated. Hepatic artery infusion with floxuridine and dexamethasone was studied in 34 patients with unresectable primary liver tumors at Memorial Sloan-Kettering Cancer Center. Partial responses were seen in 16 patients (47.1%); time to progression and response duration were 7.4 and 11.9 months, respectively. With median follow-up of 35 months, the median survival was 29.5 months and the 2-year survival was 67%.38
The introduction of targeted agents during the last decade has led to the development of new strategies to treat HCC even in patients with underlying cirrhosis. The Sorafenib Hepatocarcinoma Assessment Randomized Protocol trial was a multicenter, phase III, double-blind, randomized trial demonstrating a significant benefit in overall survival in patients with advanced HCC treated with sorafenib versus placebo.39 Sorafenib is a multikinase inhibitor that simultaneously inhibits molecular components of the Raf-MEK-ERK and the vascular endothelial growth factor receptor (VEGFR)–1, VEGFR-2, VEGFR-3, and platelet-derived growth factor receptor–β signaling pathways.39 Thus, it inhibits both tumor growth and angiogenesis. In this study, patients receiving sorafenib had almost a 3-month longer median survival and time to radiologic progression (10.7 versus 7.9 months and 5.5 versus 2.8 months, respectively). This study was limited to patients with well-preserved liver function (Child-Pugh class A), but appeared to be safe in this population. A second phase III study confirmed these results in Asian patients.40
Based on the results of these trials, sorafenib has become the standard of care for selected patients with Child-Pugh class A liver function with disease that is unresectable, extensive, and not suitable for liver transplantation; local-only disease in patients who are medically inoperable; or metastatic disease.41
Nonsurgical Local Therapy Options
Given that the majority of primary liver tumors are not amenable to surgical therapy, a number of other modalities have been examined. The hepatic artery is a site of considerable interest, as it is generally the principal supply for the major portion, particularly centrally, of HCC tumors.22 Minimally invasive procedures such as hepatic artery ligation or embolization have been used with HCC, but are contraindicated in the presence of severe cirrhosis, portal vein invasion, or thrombosis.7 Embolization can have short-lived effects because of recanalization. Although some studies suggest an improvement in survival with chemoembolization, randomized trials have failed to confirm this.22,42,43 Cryosurgery has been attempted in small series. Ethanol injections, both percutaneously and intraoperatively, have been used for small primary tumors and for recurrent nodules. Necrosis at the site readily occurs, although hepatic recurrences elsewhere remain the rule. It seems to work best for lesions smaller than 3 cm and is a common technique in Japan.7 Hot saline has also been used for intralesional injection. A recent report describes no recurrences (with limited follow-up) and pathologic demonstration of no residual tumor in the few patients who underwent post-treatment biopsies.44
Radiation Therapy Options
Early on, researchers determined that low doses of anterior-posterior–posterior-anterior hepatic irradiation were ineffective in controlling gross HCC disease and that higher doses of whole-liver irradiation resulted in high rates of radiation hepatitis. Stevens45 states that 75% of patients treated with 40 Gy or greater to the whole liver will develop liver dysfunction and reports Finney’s study46 showing that 4 of 52 patients treated with more than 55 Gy had fatal hepatitis. These widely quoted early results have resulted in RT being placed on the “palliative” list of potential HCC therapies. More recent work has emphasized that, although high doses of radiation cannot be administered to the whole liver, this does not necessarily imply that RT has no value, but rather that classic approaches to portals and fractionation must be abandoned and that innovative techniques should be evaluated. These fall into five categories: technical innovations designed to minimize normal hepatic irradiation, new fractionation protocols, new modalities, the use of radioisotopes, and the use of multimodality treatment.
With the development of three-dimensional conformal RT, high-dose, partial liver irradiation was introduced for patients with unresectable liver tumors.47–49 Investigators at the University of Michigan have reported a phase II study of three-dimensional conformal RT and concurrent intra-arterial floxuridine chemotherapy for unresectable hepatobiliary disease. They treated 128 patients with unresectable HCC (n = 35), IHCC (n = 46), or colorectal hepatic metastases (n = 47).50 Doses ranged from 40 to 90 Gy (median, 60.75 Gy) and were based on the amount of normal liver tissue, evaluated by dose-volume histograms, which could be effectively excluded from the fields to a maximum risk of radiation-induced liver disease (RILD) of 10% to 15%. At a median follow-up time of 16 months (26 months in patients who were alive) the median survival was 15.8 months and the actuarial 3-year survival was 17%. These numbers exceed those quoted for radiation or regional chemotherapy alone and match most surgical series, and should serve as the basis for future studies using dose escalation and radiation sensitizers. Overall toxicity was acceptable, with only 38 patients (30%) developing toxicity of grade 3 or higher. Five patients developed RILD, one of whom died.
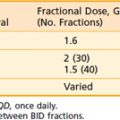
Alternative fractionation schemes have also been introduced in the treatment of primary liver tumors. Both hyperfractionation (lower dose per fraction, twice-daily treatments) and hypofractionation (high dose per fraction in fewer fractions) have been applied to improve response rates in liver malignanceis. The rationales for hyperfractionation are the rapid doubling time of HCC tumors (estimated at 41 days) and the shortened treatment times. Hyperfractionation was used in the University of Michigan series.47–50 In a nonrandomized study by the Radiation Therapy Oncology Group (RTOG),51 treatment to the whole liver was given to 194 patients with advanced HCC (70% to 80% had metastases or had failed prior treatment). Conventional fractionation (3 Gy four times per week to 21 Gy) was used in 135 patients, whereas 59 received 24 Gy in 1.2-Gy fractions twice a day. Concurrent doxorubicin and 5-FU were given intravenously every other day to both groups. The response rate for both was dismally low (≅20%) and unaffected by fractionation scheme. Both acute esophagitis and thrombocytopenia occurred significantly more often in the group that received twice-daily radiation.
With improved tumor localization techniques and motion-management modalities, hypofractionation using image-guided RT has been evaluated in the treatment of primary liver tumors. Focal, high-dose stereotactic body RT (SBRT) was evaluated in a phase I study of 41 patients with primary liver tumors, the majority of which were HCC. Dawson and colleagues49 prescribed a variable dose (24-54 Gy) given over six fractions; the dose depended on the volume of liver irradiated and the estimated risk of liver toxicity using a normal tissue complication model. Grade 3 elevation of liver enzymes was seen in five patients (12%). No RILD or treatment-related grade 4 or 5 toxicity was seen within 3 months of SBRT, and overall clinical outcomes were better than expected for this high-risk population. This study was limited to patients with Child-Pugh A or less cirrhosis. However, the majority of patients with unresectable HCC have more advanced cirrhosis and the underlying liver dysfunction must be considered when delivering any type of liver irradiation. There are emerging data that dose-volume constraints may be used in cirrhotic patients receiving conformal RT for HCC.52,53 Such guidelines have been shown to allow safe delivery of relatively high doses of conformal RT for HCC.52 These guidelines were used in a phase II study that confirmed three-dimensional conformal RT could be used safely and effectively for patients with tumors smaller than 5 cm and Child-Pugh class A or B cirrhosis.53 SBRT appears to be a promising alternative for patients with unresectable primary liver tumors. This modality may prove to be an option not only for definitive treatment of primary liver tumors, but also as a bridge to transplant for HCC patients.
Hypofractionated, focal RT using charged particles has also been studied, particularly in Asia. In small, nonrandomized studies, high-dose RT delivered with protons has been shown to result in acceptable local control and survival rates for unresectable primary liver tumors.54–56 A recent prospective study of 51 patients treated with proton RT delivered 66 GyE in 10 fractions to patients with one to three HCCs (≤10 cm).55 Of these patients, 20% were in Child-Pugh class B. The 5-year local control and survival were 88% and 39%, respectively. Among patients with solitary tumors and Child-Pugh class A liver function, 5-year survival was 46%. These results are similar to those obtained after surgery. Liver function remained stable or improved in 84% of patients, and no RILD was observed. Investigators at Loma Linda Hospital reported the results of their phase II study in which 34 HCC patients were treated with 63 Cobalt Gy Equivalents (CoGyEq) in 15 fractions using protons, resulting in a 2-year local control rate of 75%.57 Six patients underwent liver transplantation between 6 and 16 months after completion of RT, two of whom had no residual tumor. These newer modalities may be able to minimize normal tissue effects via precise dose localization, and should be investigated further.
Selective internal RT (SIRT) or radioembolization using Yttrium-90 (90Y)–labeled microspheres has gained interest in recent years. 90Y microspheres have several advantages as a potential therapy for liver lesions, including the selective delivery of the radiolabeled microspheres via the hepatic artery as well as the short depth dose afforded by 90Y, a beta emitter. A meta-analysis of SIRT using either resin or glass microspheres demonstrated that, for HCC, a treatment response (complete response, partial response, or stable disease) was seen in 89% for resin microspheres and 78% for glass microspheres.58 In a large, multi-institutional, retrospective review of 515 patients who received 680 treatments of SIRT, stable disease was seen in 76.8%, a partial response in 9.5%, a complete respone in 4.5%, and progressive liver disease in 9%. The treatment was well tolerated with an incidence of RILD in only 4% and non-liver grade 3 or higher toxicity in 6% (gastritis or gastric ulceration).59
Lastly, recent data suggest that the use of combined RT and embolization may give promising results. Seong et al.60 from South Korea evaluated 27 patients who had failed transarterial chemoembolization (TACE; failure judged by incomplete tumor filling of lipiodol adriamycin on angiogram or CT), and went on to receive RT (51.8 Gy ± 7.9 Gy). They found objective responses in 67% of patients, a median survival from RT start of 14 months, and 1-year overall survival of 85%.60 In a separate review, Seong et al.61 evaluated 30 patients treated with TACE followed by planned RT, and noted a 63% objective response rate and a median survival of 17 months. Cheng et al.62 reported similar results, with a median survival of 19.2 months in patients treated with combination radiation and TACE.
Metastases
Radiation offers good palliation for extrahepatic metastases from primary liver tumors. Anecdotal reports have revealed excellent relief of metastatic bone pain (80% to 100%), and Chen and colleagues24 noted that good responses to radiation were seen in 33 patients with metastatic HCC with skin, nodal, pulmonary, and brain lesions, although recurrences were seen.
Follow-Up
After any treatment for primary liver tumors, patients should be closely followed with serial tumor markers (AFP titers or CA19-9), contrast-enhanced CT or MRI, and chest x-ray examinations. Ikeda and co-workers25 make a convincing case for aggressive treatment of recurrences with some of the best survival rates reported in the literature.
Extrahepatic Cholangiocarcinoma
Epidemiology and Etiology
EHCC, or cholangiocarcinoma of the extrahepatic bile ducts, refers to malignancies of the extrahepatic biliary tree, commencing from the biliary confluence (hilum) extending to the common hepatic duct. There are approximately 4500 new cases per year in the United States, with the peak incidence in the eighth decade of life.63,64 EHCC is slightly more common in men, but the exact ratio varies from study to study. A host of predisposing factors have been proposed, as outlined in Table 39-2. The most widely accepted associations are with liver flukes, ulcerative colitis, and congenital (choledochal) biliary cysts. The most common risk factor in the West is primary sclerosing cholangitis, an inflammatory condition often associated with ulcerative colitis.
Table 39-2 Carcinoma of the Bile Duct: Proposed Causal Factors and Associations
Pathologic Conditions
More than 90% of EHCC are adenocarcinomas of varying differentiations. Subclassifications include papillary, nodular, and sclerosing. The World Health Organization histologic classification5 describes three grades and six variations of adenocarcinomas (papillary, intestinal type, mucinous, clear cell, signet ring, and adenosquamous). The remaining 10% of bile duct lesions include squamous cell, mucoepidermoid, granular cell, small cell, adenosquamous carcinoma, leiomyosarcoma, rhabdomyosarcoma, cystadenocarcinoma, carcinoid, Kaposi sarcoma, angiosarcoma, melanoma, and lymphoma.
Clinical Presentation
Initial signs and symptoms include jaundice, pruritus, right upper quadrant or epigastric pain, anorexia, weight loss, nausea, fever, hepatomegaly, and a right upper quadrant mass (Table 39-3). In end-stage disease, patients may have a periumbilical mass, massive ascites and limb edema, a rectal shelf, or supraclavicular lymphadenopathy. When initially diagnosed, EHCC tumors usually appear relatively small and circumscribed; patients rarely present with signs of overt metastatic disease. The bile duct tree is variably involved; 40% to 60% involve the hilus (upper third; Klatskin tumors), 10% to 15% involve the middle third, and 15% to 25% are in the distal third.64,65 Less than 10% of patients present with multifocal or diffuse involvement of the biliary tree.64–66 Although prognosis has historically been considered worse for hilar cholangiocarcinoma than for distal tumors, this is thought to represent the relatively later presentation and failure to institute effective therapy. Recent studies suggest that location in the biliary tree has no effect on survival, provided that complete resection can be performed.65 Approximately 30% of patients have lymph node involvement at presentation65,67; the lymph nodes are a frequent site of recurrence owing to the extensive lymphatics in the bile duct submucosa. Intrahepatic involvement is extremely common at the time of failure, but is usually contiguous with the bile duct lesion itself. Metastases appear in the peritoneum, distant liver, bones, thoracic lymph nodes, and surgical or drain incisions65,68; but often, lethal local recurrence develops before systemic spread is clinically manifested.
Table 39-3 Carcinoma of the Bile Duct: Signs and Symptoms
| Symptoms | |
| Signs | |
| Advanced disease |
Diagnostic Studies
Local imaging studies generally begin with ultrasonography to evaluate jaundice, and then proceed to CT scans, which often show dilated intrahepatic ducts and a normal-appearing extrahepatic biliary system. Historically, percutaneous transhepatic cholangiography, ERCP, and angiography were considered standard investigations; currently, however, at Memorial Sloan-Kettering Cancer Center, MRCP, CT, and duplex ultrasonography have replaced these invasive tests and can often provide the same information with less risk to the patient.65 High-quality CT scans can provide indispensable information regarding vascular involvement, level of obstruction, and extent of liver atrophy. Similarly, in the hands of an experienced operator, duplex ultrasonography can provide information not only about vascular invasion, but also about tumor extension within the bile duct and adjacent tissues. MRCP can provide information about the location of the tumor, level of biliary obstruction, presence of isolated ducts, patency of the hilar vascular structures, and the presence of nodal or distant metastases. Invasive cholangiography continues to have multiple uses, however, because it can serve to localize the tumor, act as a biopsy route, and allow for stent placement for biliary drainage or brachytherapy catheters; the need for an invasive procedure needs to be weighed with the risks of biliary intubation. Each modality can serve to pinpoint with considerable accuracy a site of stricture or obstruction, or intraductal disease extent. The primary metastatic study is a chest CT scan.
A tissue diagnosis is preferred, because several other conditions can mimic bile duct cancers, particularly sclerosing cholangitis. Cytologic examination is accurate approximately 60% to 75% of the time.6,65 The optimal method for obtaining tissue is controversial, however. Different institutions prefer brushings, CT-guided needle biopsies, or controlled open procedures. Buskirk and coworkers69 have shown a 37% incidence of peritoneal seeding if there is violation of the lesion, and they recommend the use of transabdominal fine-needle biopsies following stent placement.
Staging
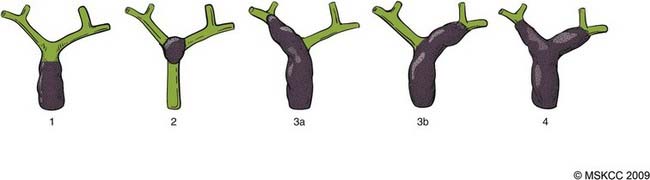
EHCC can be classified by location in the biliary tree as hilar (at or above the bifurcation of the hepatic duct) and distal (below the bifurcation, including the intrapancreatic bile duct).70 Tumors of the hepatic duct bifurcation are classified into four types according to the Bismuth classifciation71 shown in Fig. 39-4.
The AJCC TNM staging classification for extrahepatic bile duct carcinoma is provided in Table 39-4. It has limited applicability in evaluating the older medical literature because of its modified and inconsistent use. In addition, pathologists apply and report these criteria inconsistently; biopsy-only specimens can include insufficient tissue for full T staging; and lymph nodes are often not resected, even during curative procedures. Lastly, because this staging system is based on pathologic criteria, it has little value in preoperative staging and prediction of resectability.
Table 39-4 Classification of Carcinoma of the Bile Duct
Therapeutic Modalities
Surgery
The primary curative modality for EHCC tumors is surgery. However, resectability rates are low and tumor location can be a critical factor in resectability, applicable surgical procedure, and survival. As preoperative diagnostic expertise increases, the ensuing ability to safely perform complete resections grows as patient selection improves, particularly with Klatskin tumors.64,72 Contraindications to “curative” surgical resection include hepatic duct involvement up to secondary biliary radicles bilaterally, encasement or occlusion of the main portal vein proximal to its bifurcation, atrophy of one hepatic lobe with encasement of the contralateral portal venous branch or with contralateral involvement of secondary biliary radicles, distant metastases (peritoneum, liver, lung), and significant medical comorbidities.65 EHCC resections demand considerable skill and experience on the part of the surgeon. Klatskin tumors can require lobectomy. Distal bile duct tumors are often treated with a Whipple procedure (pancreaticoduodenectomy). The primary goal of palliative procedures is to improve the quality of life through resolution of jaundice and pruritus, prevention of infection, and improvement in pain. Options include palliative debulking, external or internal stent placement, and bypass operations, including hepaticojejunostomies for proximal disease. Palliative procedures are accompanied by high 30-day mortality rates and short survivals with inconsistent improvements in quality of life.
Survival rates after any surgical resection, even curative ones, are low. Median survivals after gross total resection and palliative surgery have been remarkably consistent during the past decade, with values of 17 to 42 months versus 7 to 10 months.64,66,72,73 Likewise, Boerma,74 in an analysis of more than 1000 patients treated worldwide, found a mean survival of 21 versus 11 months.
During the past 20 years, increasing extended hepatic resections, especially for patients with hilar cholangiocarcinoma, has led to an increase in the percentage of negative margin resections, and an observed improvement in survival in some series.65 Memorial Sloan-Kettering reported on 225 patients presenting with hilar cholangiocarcinomas. Of these patients, 65 had unresectable disease; 160 patients underwent exploration with curative intent; and 80 patients underwent resection: 62 (78%) had a concomitant hepatic resection and 62 (78%) had an R0 resection (negative histologic margins).75
Wide variations in morbidity and mortality are seen because of variabilities in tumor site, disease extension, medical condition, referral patterns, and institutional expertise. The mortality and morbidity rates after curative resection, however, are relatively high when compared with liver resections performed for other indications. Even in large referral centers, mortality rates can be up to 5% to 10%. Postoperative complication rates are also high (39% at Johns Hopkins,76 25% at the University of California–Los Angeles77), and can include wound infection, liver or subphrenic abscess, cholangitis, pulmonary embolus, gastrointestinal hemorrhage, and small bowel obstruction.
Local recurrence is the most common site of failure and cause of mortality: after potentially curative resections, the local failure rate was 59% in the study by Kopelson and associates78 and 53% in the study by Cameron and associates.76
Liver transplantation has been performed at several centers.79,80 Meyer and colleagues80 reported their experience with 207 patients with unresectable cholangiocarcinomas treated with liver transplantation. The 2- and 5-year survival rates were 48% and 23%, respectively. The Mayo Clinic recently published their results with neoadjuvant chemoradiotherapy followed by orthotopic liver transplantation. Of 148 patients enrolled on the protocol, 90 completed treatemnt. Overall, 1-, 3-, and 5-year patient survival was 82%, 63%, and 55%, respectively; 1-, 3-, and 5-year survival after orthotopic liver transplantation was 90%, 80%, and 71%.79 However, the reported high incidence of recurrence both in the allograft and in distant organs after transplant for EHCC suggests that this should not be considered a standard treatment option.65,80
Chemotherapy
The use of chemotherapy has been described in multiple, small, single-institution studies. A pooled analysis of 112 trial arms and 2810 patients evaluating the activity of systemic chemotherapy in advanced biliary tract cancers was recently published.81 The pooled response rate (complete response or partial response) with all chemotherapy agents was 22.6% and the pooled tumor control rate (complete response, partial response, or stable disease) was 57.3%. Gemcitabine and platinum compounds exhibited the highest activity with response rates of 25% and 30%, respectively. Combinations of chemotherapy seems to be more effective than single agents in the palliative setting. For those patients with completely resected disease, there does not appear to be a role for adjuvant chemotherapy; however, phase III trials are needed to further evaluate the role of adjuvant chemotherapy.82 An ongoing intergroup study is examining the role of adjvuant gemcitabine and capecitabine followed by RT and capecitabine after resection of EHCCs.
Radiation Therapy
Historically, the use of external-beam RT was largely limited to unresectable, recurrent, or gross residual disease treated in the pre-CT era, and this led to the conclusion that extrahepatic bile duct carcinomas were radioresistant. There is, however, no evidence that bile duct tumor cells have an inherent radioresistance, but rather that the doses of radiation generally administered have been limited by the surrounding structures. Indeed, some recent reports indicate that radiation limited to relatively small fields but reaching higher dose levels, combined with more modern surgical approaches and techniques, may have a positive effect on outcome.67,68,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree