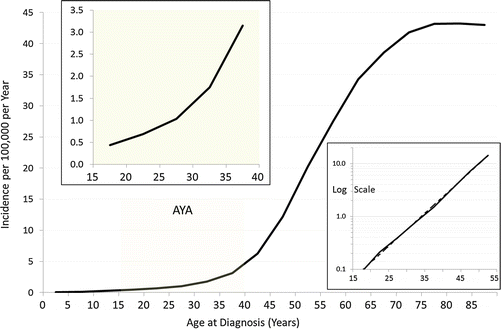
Fig. 17.1
Incidence of renal cell carcinoma by age, sex (left panel), and extent of disease (right panel) at diagnosis, 2000–2011, SEER18. The left panel inset has a log scale for the y-axis and exponential regressions (dotted lines)
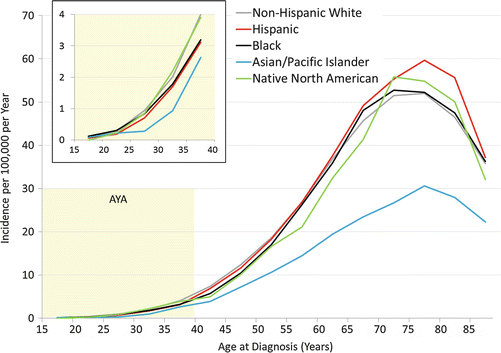
Fig. 17.2
Incidence of renal cell carcinoma by age and race/ethnicity, 2000–2011, SEER18
17.1.3.2 Incidence Trends
In older adults, there has been a steady increase in incidence since at least 1975 (Fig. 17.3, right panel). In AYAs, the incidence remained the same until 1992 (Fig. 17.3, left panel), when a marked increase in incidence began to be seen. Since 1992, the rate of increase in incidence has been much greater in AYAs than in older adults (Fig. 17.4, upper panel), especially in females and particularly in females 15 to 25 years of age (Fig. 17.4, lower panel). The root cause of these changes is unknown, but this pattern would be seen if there had been a recent increase of exposure of the population to an environmental agent which increased risk.
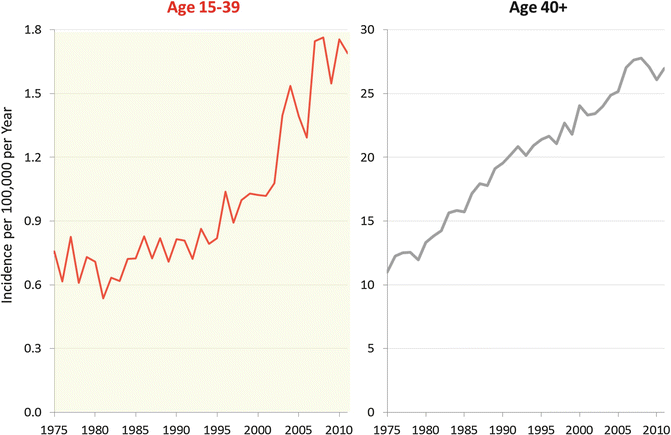
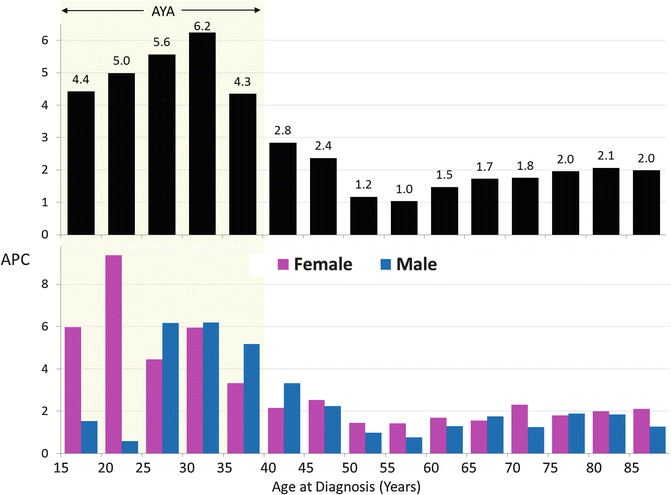

Fig. 17.3
Annual incidence of renal cell carcinoma by age, 1975–2011, SEER9

Fig. 17.4
Annual Percent Change (APC) in incidence of renal cell carcinoma by age and sex, 1992-2011, SEER13
17.1.4 Clinical Presentation and Diagnosis
The diagnosis of renal tumors in AYAs is challenging. The classic triad of local symptoms of the primary malignant renal tumor (hematuria, flank mass, and abdominal pain) may be absent or appear late. Metastatic disease may present with local symptoms from metastatic sites or with generalized symptoms, like weight loss, cachexia, fevers, and sweats. Symptoms are thus nonspecific and clinical examination is relatively insensitive at detecting a small tumor. The differential diagnosis is likely to be dominated by nonmalignant conditions, first of all urinary tract infections. Inevitably this contributes to a delay in diagnosis for many patients. For example, hematuria in this age group is usually due to urinary tract infection. In older patients, in whom malignant disease is more likely, hematuria is routinely a trigger for full investigation to exclude malignant disease by imaging, cytology, and endoscopy. However, in the younger patient, empiric treatment with a suitable antibiotic is reasonable for the first episode, although assessment should include microbiological examination of the urine. Patients with recurrent or persistent hematuria, especially when no infective agent is identified, should be referred for further investigation including a pelvic examination, renal tract ultrasound, urine cytology, and eventually cystoscopy.
If a renal tumor is suspected, diagnostic imaging studies play a central role in the evaluation of initial extent of disease and for planning surgery or monitoring the response to therapy, in cases in which a systemic preoperative treatment is indicated. Parameters that should be carefully evaluated are the extent of the tumor within and behind the kidney, involvement of the contralateral kidney, the presence of intravascular tumor thrombosis (renal and cava veins), and the presence of retroperitoneal lymph node involvement.
The initial radiographic study is usually an abdominal ultrasound examination. Cross-sectional imaging, either contrast-enhanced computed tomography or magnetic resonance imaging of the abdomen, is then recommended to further evaluate the nature and the extent of the renal mass. Despite thorough clinical and radiographic evaluation, some renal masses will remain indeterminate, and their management is subject to individual clinical opinions. Careful correlation of clinical and imaging findings may facilitate the preoperative diagnosis of renal lesions; however, a percutaneous needle biopsy might be indicated to correctly plan the following steps toward diagnosis and treatment, especially in this age group when tumors different from Wilms’ tumor are more frequently seen.
17.1.5 Treatment
Apart from Wilms’ tumor, which is usually highly chemosensitive, surgery remains the mainstay of curative therapy for patients with renal tumors in this age group. Complete surgical removal at the earliest possible occasion remains the approach most likely to lead to a full recovery.
17.1.5.1 Wilms’ Tumor (WT)
The literature suggests that outcome for AYAs with WT is worse than that for pediatric patients [9]. There are various possible explanations for this observation. In the UKW3 trial for patients with WT, increasing age was an independent poor prognostic factor (although the age categories studied were less than 2 years, 2–4 years, and greater than 4 years, respectively, and very few patients were older than 15 years) [10]. Since WT is very sensitive to chemotherapy, children presenting with advanced-stage or metastatic disease are curable, with long-term survival over 70 % [11]. The possibility that chemosensitivity may reduce with age is supported by case reports of older patients who failed to respond to treatment [12, 13], but the consensus remains that pediatric chemotherapy protocols should still be followed where possible and can be successful in some cases [12, 14]. In the largest series in the literature of adult WT, the overall survival of 17 patients was 67 % [15]. Of note, a significant proportion of AYAs diagnosed with WT are delayed in starting postoperative chemotherapy for various reasons, and this seems to represent a negative prognostic factor [9]. In addition, AYA series on WT report a higher incidence of advanced-stage disease (stage III or IV) than that noted in pediatric series. Cytogenetic studies of a single case suggest that there may be different molecular lesions in adult cases [16] and this may also explain different response to systemic therapy. However, the consensus remains that AYA patients should be treated according to (pediatric) protocols developed by the International Society of Pediatric Oncology (SIOP) or the Children’s Oncology Group (COG).
17.1.5.2 Renal Cell Carcinoma
Though rare in adolescents, there are important questions regarding the best treatment approach and accurate pathologic classification. There is increasing evidence to suggest that RCC arising in adolescents differs from adult cases [1, 17, 18] (reviewed in [19]). Importantly, age-related differences may mean a significantly dissimilar response to therapies between different age groups.
Surgical treatment aiming for complete tumor resection remains the mainstay of therapy, radical nephrectomy being the gold standard. Since the incidence of RCC is an increasing AYAs (Fig. 17.3, right panel), primary radical nephrectomy should be regarded as the initial treatment in this age group or biopsy considered in cases of doubtful diagnosis. While elective partial nephrectomy is being used in adults based on clearly defined criteria, caution is needed in transferring this approach directly to adolescents because of the potentially different intrarenal behavior of translocation RCC. However, since surgical approaches that preserve a healthy renal parenchyma are advocated for adults with small-volume tumors and have demonstrated a good long-term oncological outcome, it is reasonable to assume that they should be studied in adolescents as well. The question of the most adequate extension of retroperitoneal lymph node dissection remains controversial, especially in cases without clinical evidence of lymph node spread. It is noteworthy that, while lymphatic spread by RCC certainly makes the outcome worse in adults, the same is probably not true for younger patients. The therapeutic value of complete retroperitoneal lymph node dissection is still controversial, especially in patients without suspected nodal involvement, be they adults or adolescents [20]. However, given that current adjuvant therapies are not curative, it might be worth emphasizing whether a more aggressive approach to radical lymphadenectomy should be advocated to achieve surgically complete disease resection in these patients. Since the incidence of RCC increases with age throughout childhood and equals or exceeds that of WT during the second decade of life, a pragmatic recommendation might be to discuss the indication for lymph node dissection with the surgeon instead of sampling, in adolescents.
The landscape of systemic therapies in RCC has recently been changed by the introduction of drugs targeting tumor-related angiogenesis and signal transduction [21–23]. These are the multitargeted receptor tyrosine kinase inhibitors (sorafenib, sunitinib, pazopanib), the inhibitors of the mammalian target of rapamycin (mTOR) pathway (temsirolimus, everolimus) and the antiangiogenic monoclonal antibody bevacizumab.
In previous years, research focused largely on biological therapies, with tantalizing results in small series that suggested that the disease is amenable to manipulations of the immune system with cytokines. Both interleukin-2 and interferon have previously shown a small benefit on progression-free interval, yet are now limited to very selected groups of adult patients. In the adult, treatment of metastatic RCC using conventional cytotoxic chemotherapy has not been associated with clear evidence of significant benefit on outcome.
Data on the use of antiangiogenic agents in RCC is largely confined to the older adult population, and studies on adolescents are largely limited to retrospective case reports or series collected at only one or a handful of institutions [7, 17]. It is worth noting that the largest clinical efficacy trials on targeted molecules have been conducted on clear cell subtype of RCC. Importantly, von Hippel-Lindau gene inactivation has been identified as a main driver in clear cell RCC, with somatic mutations or hypermethylation being present in over 90 % of cases, enabling the rationale for therapies contrasting tumor angiogenesis in the clear cell subtype of tumors. But while targeted drugs have become the standard of care for adult metastatic RCC, there are currently few data on their effectiveness in pediatric translocation RCC, and their use should be considered for patients with unresectable metastatic or advanced-stage disease [24].
The standard of first-line care for adult metastatic RCC is based on administering tyrosine kinase inhibitors targeting the VEGF receptor, while mTOR inhibitors are generally used as a second-line treatment when tyrosine kinase inhibitors fail or as first-line therapy for poor-risk patients [25–27]. On the other hand, the utility of these therapies in the adjuvant setting remains to be seen. This uncertain benefit, together with their toxicity and the relatively better outlook for adolescents with completely resected involved lymph nodes, supports the decision not to use adjuvant therapies in younger patients. What might be recommended for adolescents with metastatic RCCs is sequential treatment with VEGF pathway-targeted therapies, optimizing the results in terms of efficacy and safety. In the final decision as to the best approach to RCC, it should be emphasized that, despite transient responses to targeted therapies, no durable complete remissions have been obtained without chronic, often multiline treatments in most patients. Initial nephrectomy may also be useful even in the face of metastatic disease, and selected patients may benefit from metastasectomy of the lung and even the brain.
17.1.6 Outcomes
17.1.6.1 Survival
Previously published data suggest that the overall survival rate for adolescents with RCC (irrespective of stage) is around 50–60 %, with outcomes worsening with older ages [17, 28–30]. Patients with tumors localized in the kidney, with or without regional lymph node spread, have a good prognosis, while the outcome remains poor for patients with distant hematogenous metastases.
Geller et al. recently reported on 120 consecutive young patients with RCC (median age was almost 13 years), prospectively registered on the Children’s Oncology Group study AREN03B2, forming a large prospective series of well-characterized pediatric tumors for the first time [18]. The authors can confirm that RCC in adolescents typically presents at advanced stage, with Xp11.2 translocation type being the most common (47 %), that lymph node involvement is frequent (48 %) – even among patients with small associated primary tumors – and imaging sensitivity for the detection of lymph node metastases remains poor. Noteworthy, as these tumors have only been recently recognized, their natural history is poorly defined, and extended follow-up is recommended because, regardless of the age of the patient, translocation RCCs seem to have the potential to metastasize also after many years.
Updated analysis of SEER data for this report shows that during 2000–2011, the 5-year RCC-specific survival rate was inversely proportional to age at diagnosis above age 30 (Fig. 17.5) [52]. Below age 30, however, the reverse was true, and for 20- to 25-year-olds, the 5-year rate was lower than in middle-aged adults (Fig. 17.5). Renal cell carcinoma that presents with localized disease has a >90 % 5-year cancer-specific survival at all ages up to 75 years (Fig. 17.6). Among AYAs, it is >95 % (Fig. 17.6). AYAs with metastatic disease at diagnosis have a significantly better 5-year RCC-specific survival than older patients (Fig. 17.6). The reverse appears to be true for regional disease at diagnosis (Fig. 17.6). Blacks have the lowest 5-year renal cell carcinoma-specific survival among the major races/ethnicities in the United States, especially among AYA patients (Fig. 17.7).
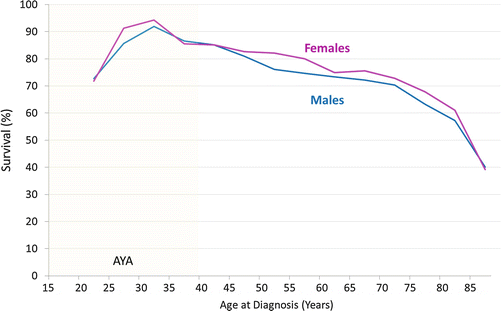
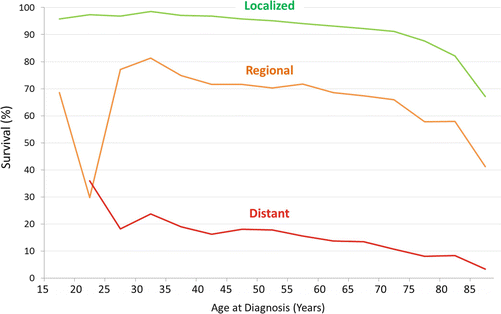
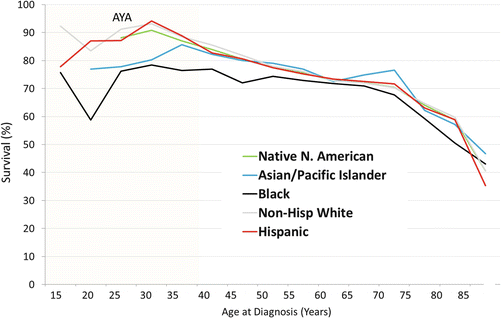

Fig. 17.5
A 5-year RCC-specific survival by age (15+ years) and sex, 2000–2011, SEER. Age subgroups with <25 patients are not included (age <20)

Fig. 17.6
A 5-year renal cell carcinoma-specific survival by stage and age, 2000–2011, SEER. Age subgroups with <11 patients are not included (age <20)

Fig. 17.7
A 5-year head/neck cancer-specific survival by age (>15 years) and race/ethnicity, 2000–2011, SEER. Age subgroups with <9 patients are not included
17.1.6.2 Survival Trends
Both male and female AYAs have had an improvement in their RCC survival rate similar to that in older adults, and both have a 5-year cancer-specific survival rate that is about 15 % greater than in corresponding older adults (Fig. 17.8).
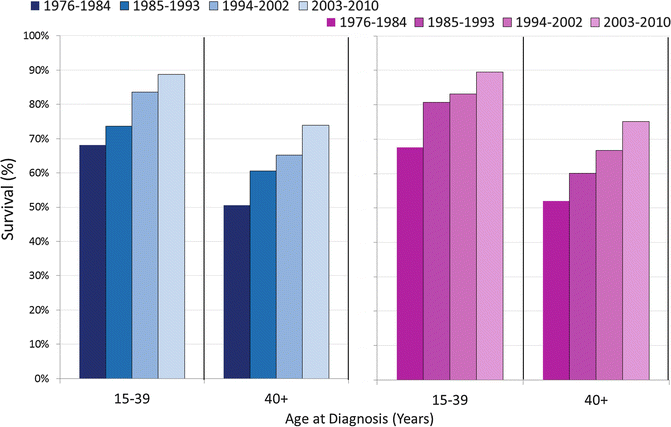

Fig. 17.8
A 5-year renal cell carcinoma cancer-specific survival by age and era, 1976–2010, SEER9
17.1.7 Summary
There are significant challenges in managing adolescent or young adult patients with renal malignancies. Diagnosis is difficult and may be delayed, but as soon as a malignant diagnosis is confirmed, the patient and their family should be referred without delay to an institution that can provide both the highly specialized tumor-specific multidisciplinary team to deliver treatment and, ideally, one where age-specific support services are available.
Confirmation of the diagnosis is the first crucial step. Expert histopathologic review should be performed on all tumors, since the chance of an unusual histology in this age group is high. Treatment planning should involve surgical, radiation oncology, and medical oncology input.
The overall survival rate for AYAs with RCC is around 50–60 %, with outcome worsening with older patients [17, 28–30]. Patients with tumors localized in the kidney, with or without regional lymph node spread, have a good prognosis, while the outcome remains dismal for patients with distant hematogenous metastases.
Treatment guidelines developed for the management of adult patients should be assessed carefully before extrapolating them to a different age group, but in the absence of good data to support different approaches, these are still the best recommendations for care in many cases. More research is required into the etiology (in which genetic susceptibility may play a greater part), natural history, and response to therapy in the unusually young patient with these forms of cancer.
17.2 Bladder Cancer
17.2.1 Biology, Pathology, Etiology
In the adult population, the commonest cancer of the bladder is transitional cell carcinoma (TCC) of the urothelium, which may also present in the urothelium of the renal pelvis and ureter. Clearly, this reflects the fact that this tissue is exposed to carcinogens in the urine. Polymorphism with regard to key protective pathways may account for increased risk [31] and may be implicated in patients with a very young age at diagnosis. Normal urinary physiology is also protective, and young patients who require bladder augmentation due to neurological disorders are at a higher risk of developing TCC [32]. Excess incidence of urinary cancer, including very young patients, has been detected in studies of areas affected by heavy pollution [33]. In addition to TCC, rare histological variants are also found, and one might expect these tumors to account for a higher proportion of cases in the much younger patient population [34].
In one of the few published series of adolescent patients diagnosed with bladder cancer, all of the patients had well-differentiated and low-stage tumors [35]. This may be due to “lead-time effect” where early diagnosis of a tumor whose natural history is to progress from low grade to high grade and from localized to metastatic will lead to an association between young age at diagnosis and low grade and lower stage. This hypothesis is also supported by a very large epidemiological study from the National Cancer Database, which demonstrated an association between young age and low stage [36]. In a more recent series, younger age (<40 years) was confirmed to be associated with good overall survival due to a higher proportion of low-stage, low-grade tumors. However, in those patients with high grade, there was a higher risk of recurrence [37].
17.2.2 Epidemiology
17.2.2.1 Incidence
In the updated SEER analysis covering 2000–2011, the incidence of bladder cancer had a distinct sigmoid relationship with age (Fig. 17.9) [51]. Males had a greater incidence than females, but this difference was most marked after the age of 30 (Fig. 17.10). The vast majority of bladder cancers in AYAs were localized at diagnosis, and the remainder were regional with very few cases of AYAs presenting with distant metastases (Fig. 17.11). In addition to an association with gender, a significant association was seen with ethnicity: non-Hispanic white people being at significantly more risk than others (Fig. 17.12).