43 Cancer of the Kidney
Epidemiology and Risk Factors
In 2008, there were 54,390 new cases and 13,010 deaths in the United States. According to the statistics reported by the American Cancer Society, cancer of the kidney is the seventh most common cancer in men, and the ninth most common in women.1 More than 85% of these tumors are renal cell carcinomas (RCCs), with the remainder consisting of less common tumors such as transitional cell carcinomas of the renal pelvis and Wilms tumors. Approximately 30% of patients present with metastatic disease; RCC accounts for 3% of all adult malignancies and demonstrates a slight male predominance, with 60% of the diagnoses and deaths occurring in men.
With a peak incidence in the sixth to eighth decades, RCC is a tumor of older adults, although presentation at other ages is not uncommon, especially in cases involving hereditary syndromes. The incidence of RCC is increasing, in part because of the incidental diagnosis of small renal masses (SRMs) (masses ≤4 cm) captured by the increased use of computed tomography (CT) scanning. In the past, treatment of these SRMs has been aggressive, with surgical resection rather than observation. But despite this aggressive treatment, mortality is actually increasing and is greatest in tumors larger than 4 cm. This “treatment disconnect” is likely related to the fact that the majority of these SRMs are benign (20%) or clinically indolent when malignant (70%).2,3 Surveillance of these SRMs has become an acceptable option for the compliant patient.
Numerous risk factors have been associated with the development of RCC, many of which are listed in Table 43-1. Cigarette smoking has a well-established association, with the risk of RCC increasing proportionately with pack-years of use. Patients with end-stage renal failure on long-term hemodialysis are at risk for developing acquired cystic disease of the kidney. It is this latter condition, and not hemodialysis per se, that appears to be the causal factor for the development of RCC in this subpopulation. There is accumulating evidence that chronic diuretic therapy may be a risk factor for development of RCC.2,4,5
Table 43-1 Risk Factors for Cancer of the Kidney
| Factor |
|---|
| Cigarette smoking118–120 |
| Obesity118–120 |
| Phenacetin abuse118–120 |
| Asbestos exposure121,122 |
| Leather tanning121,122 |
| Shoe repair121,122 |
| Chronic hemodialysis123–125 |
| Von Hippel-Lindau disease8,10 |
Genetic factors also appear to increase risk. In 1979, Cohen and colleagues6 reported a familial pattern of RCC that appeared to be transmitted via an autosomal dominant gene pattern. A 3;8 translocation was found in numerous family members, and all RCC cases occurred only in those members with the translocation. Pathak and colleagues7 described a second family pedigree with RCC in 1982; however, the translocation occurred between chromosomes 3 and 11 and was present only within tumor tissue. Patients with von Hippel-Lindau (VHL) disease develop retinal angiomas, hemangioblastomas of the central nervous system, and clear cell renal carcinoma. This disease is transmitted in an autosomal dominant pattern and is associated with the development of RCC in 25% to 45% of those afflicted.8–10 Cohen has shown that the human VHL gene maps to chromosome 3p25.11 Furthermore, studies analyzing the polymorphism of restriction fragment lengths have revealed 3p deletions in nonfamilial cases of RCC.
Frequent and early loss of heterozygosity in chromosome 3p has been identified in 90% or more of spontaneous clear cell RCCs.4,5 This led to the identification of a mutation in the VHL tumor suppressor gene, which results in loss of function or reduced levels of the VHL protein. This leads to deregulation of hypoxia-inducible factor (HIF)-1α, allowing it to accumulate and bind with HIF-β.7,11 This transcriptional factor complex induces expression of hypoxia-inducible genes such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which promote angiogenesis, inhibit normal tumor cell apoptosis, and eventually lead to tumor proliferation and survival.12 The identification of this key mutation has led to the development of targeted agents against the VHL pathway in the treatment of metastatic renal cell carcinoma (mRCC). Mutations in either tumor suppressor genes or proto-oncogenes have been identified in almost all histologic subtypes of RCC.8
Although the new targeted agents have greatly increased the treatment arsenal for mRCC, the best chance of cure remains early diagnosis and surgical resection. Patients with locally confined tumors have an expected 5-year disease-specific survival of nearly 90%, and 10% of patients with metastatic disease will be alive at 5 years.13 Unfortunately, the majority of patients who present with localized disease develop metastases during the course of their disease.
Anatomy
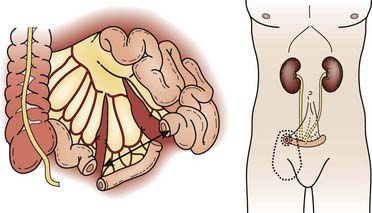
The kidneys are paired retroperitoneal organs, the medial edges of which lie parallel to the psoas muscles. Each kidney is typically 11 to 12 cm in length, corresponding radiographically to 3 to 3.5 vertebral bodies. Although there is some variation, the kidney is usually centered at L1 or L2 and extends from T12 to L3 (Fig. 43-1). Because of diaphragmatic contracture, the kidneys may move caudally by as much as 2.5 cm with inhalation. The right kidney is depressed by the liver 1 to 2 cm relative to the left and is in association with the second part of the duodenum, the right colic flexure, and the posterior portions of the right 11th and 12th ribs (Fig. 43-2). The left kidney is adjacent to the spleen, stomach, pancreas, left colic flexure, and left 11th and 12th ribs.
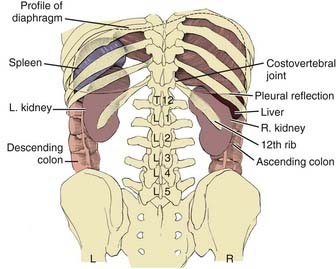
FIGURE 43-1 • Relation of the kidneys to the thorax and vertebral bodies, posterior view.
(From Hinman F Jr: Atlas of urosurgical anatomy, Philadelphia, 1993, WB Saunders, p 260.)
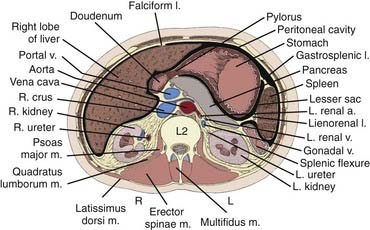
FIGURE 43-2 • Transverse section of the kidney at the L2 level.
(From Hinman F Jr: Atlas of urosurgical anatomy, Philadelphia, 1993, WB Saunders, p 262.)
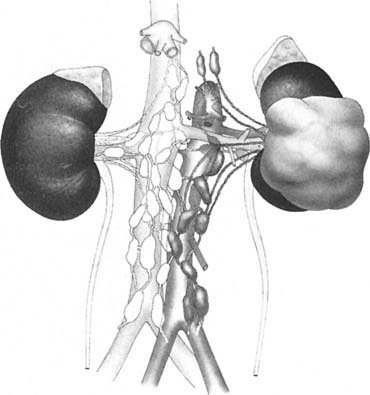
Draining lymph nodes originate within the renal hilus and follow the renal artery to the para-aortic and paracaval chains (Fig. 43-3). Regional nodal drainage extends from the crus of the diaphragm to the bifurcation of the aorta, varying somewhat with tumor laterality. For a right-sided tumor, this specifically includes the paracaval, retrocaval, precaval, interaortocaval, and preaortic lymph nodes. The para-aortic, preaortic, retroaortic, interaortocaval, and precaval lymph nodes are at risk for a left-sided tumor.
Pathologic Conditions
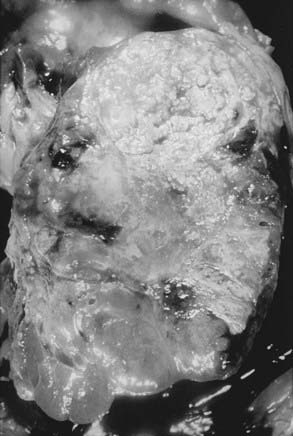
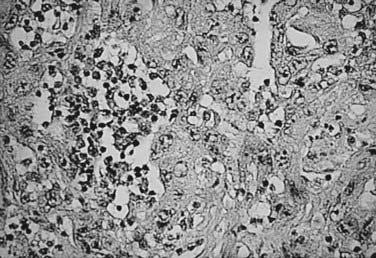
RCC is a neoplasm arising from proximal renal tubular epithelium. Historically, this tumor was erroneously considered to arise from adrenal rests within the kidney and thereby acquired the name hypernephroma, titled by Grawitz in 1883.14 Recent immunohistologic and ultrastructural analysis confirms the true renal origin of RCC,15,16 validating its more common and correct name. Gross sectioning of RCC tumors often reveals the classic yellow-tan fatty appearance with occasional focal areas of hemorrhage and necrosis (Fig. 43-4). Calcifications, fluid-filled cysts, and areas of fibrosis may also be apparent. Although not truly encapsulated, these lesions are often surrounded by a pseudocapsule because of their expansile growth pattern, which results in compression of surrounding renal parenchyma.
There has been a long-standing controversy as to the existence of a benign entity called renal adenoma, defined as lesions less than 3 cm in diameter. Although histologically indistinguishable from RCC, these lesions were considered benign since the 1938 publication of Bell’s classic paper,17 in which he described the absence of metastatic spread in lesions smaller than 3 cm. Although it is not uncommon for such lesions to be detected incidentally or at autopsy without clinical evidence of spread, there is an increased number of reports of tumors fulfilling the size criterion of renal adenoma, but with nodal and distant metastases (DMs) present.18 Furthermore, when one contemplates the natural history of a large RCC lesion, it is illogical to conclude that it had never once measured less than 3 cm. It is generally now advocated that renal neoplasms be defined as malignant or benign based on their histologic appearance, and not because of their size.
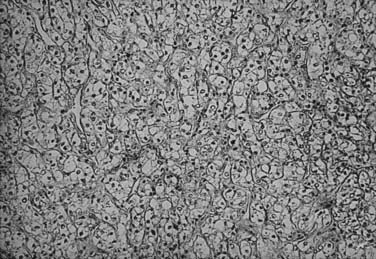
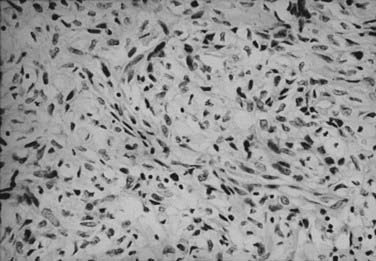
The histologic appearance of RCC is variable, with five main cell types predominating: (1) clear cell, (2) papillary type 1, (3) papillary type 2, (4) chromophobe, and (5) oncocytoma.9 Sarcomatoid can be a variant of any of these subtypes and indicates an aggressive subtype that is associated with a worse prognosis.19 Oncocytomas are considered benign (Figs. 43-5 to 43-7). Clear cell RCC is by far the most common, constituting approximately 75% of all tumors.20 Histologically, these cells contain lightly staining cytoplasm, and are rich in glycogen and lipid material. The cell of origin for clear cell carcinomas is thought to be proximal tubular epithelium. Sarcomatoid or spindle cell tumors resemble mesenchymal cells. They are frequently high grade and demonstrate an aggressive clinical course with 70% to 80% presenting with metastases.21 An individual tumor may demonstrate one or a combination of these cell types.
Clinical Presentation
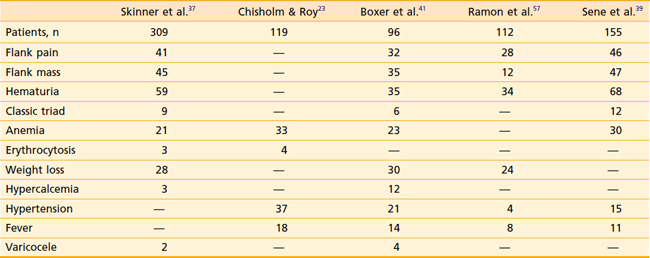
RCC has been described as “the internist’s tumor” because of the myriad of signs and symptoms that may be noted at presentation (Table 43-2), some of which are due to the direct effects of an invasive renal mass, and others of which are considered paraneoplastic in origin. Most RCCs are asymptomatic. Only 30% present with symptoms, and these cases tend to be locally advanced or metastatic. The most common symptoms are gross hematuria, flank pain, or a flank mass. Together, these symptoms compose the classic triad of RCC, which is only seen in approximately 10% of presenting patients. Whether gross or microscopic, hematuria presents primarily when the collecting system has been invaded. Flank masses resulting from RCC are firm, nontender, and homogeneous. The sudden onset of a nondraining varicocele in a male, particularly on the left, should raise the possibility of a renal neoplasm obstructing the gonadal vein at its entry point into the left renal vein.
Paraneoplastic syndromes are commonly associated with RCC.22 Anemia is among the most common, occurring in 30% to 40% of patients, and is generally considered a paraneoplastic process as it is often out of proportion to the degree of hematuria. Conversely, erythrocytosis is also a well-recognized phenomenon; however, it is much less common at around 4% to 5%. Other paraneoplastic syndromes associated with RCC include fever, cachexia, hypercalcemia, hypertension, Stauffer syndrome, amyloidosis, enteropathy, and neuromyopathy.23 Ectopic production of a parathyroid hormone–like material is responsible for the rare finding of hypercalcemia. Paraneoplastic syndromes confer a worse prognosis and do not always improve with removal of the primary tumor.
Routes of Spread
One quarter to one third of all patients are found to have DM at presentation. Furthermore, among patients treated for localized disease, the primary cause of RCC-associated death is metastatic tumor burden and not local failure (LF).24 Approximately 50% of initial metastatic sites are to the lung. The immunoprotected sites of the bone and the liver are the next most common sites of DMs. RCC goes to the brain in 2% to 10% of cases, and patients tend to have associated neurologic symptoms. Local recurrences are less common at approximately 2% or less. The common involvement of the renal vein by this tumor gives direct evidence for the early hematogenous spread commonly seen in this disease.
Staging Workup
The use of 18F-fluorodeoxyglucose positron-emission tomography (PET) in diagnosing and staging primary and mRCC is limited by its low sensitivity.25 However, its high specificity is superior to conventional imaging. Thus, PET may best be used in RCC in combination with CT to make management decisions in which the CT component provides high sensitivity and better anatomic information, whereas PET offers high specificity. This combination is useful to evaluate equivocal findings on CT such as differentiating between response to treatment and recurrence.26
Once the presence of a renal mass has been documented, a chest x-ray film and bone scan (if alkaline phosphatase is elevated) are required. If there is no evidence of DMs or impaired renal function, no further tests are required, and patients are generally brought to the operating room for definitive resection. For patients in whom a partial nephrectomy (PN) is being considered (see later discussion of indications), or a locally advanced lesion is identified, a selective renal arteriogram may be obtained to better delineate tumor extent and vascular anatomy (Fig. 43-8, Fig. 43-9, Fig. 43-10, and Fig. 43-11).27 Inferior venacavography is a useful tool for evaluating renal vein and inferior vena cava extension. Preoperative needle biopsies of the renal mass for the purpose of obtaining a tissue diagnosis may be indicated in patients with evidence of metastatic disease, but they are not required in a patient who is a candidate for definitive resection. Other complementary radiologic tests include ultrasonography, magnetic resonance imaging (MRI), and percutaneous puncture of a renal cyst.
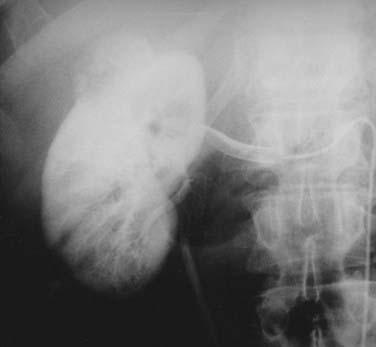
FIGURE 43-9 • Selective renal arteriogram of the patient whose scan is shown in Fig. 43-8. Note the highly vascular appearance of these tumors.
Staging Systems
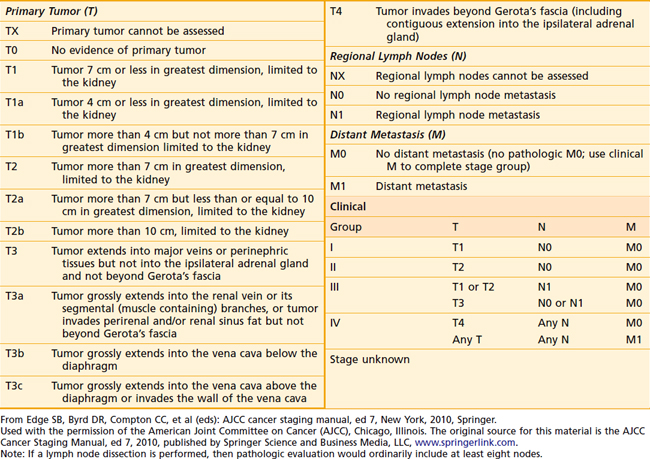
One of the oldest and most commonly used staging systems is the Robson classification system (Table 43-3), which was proposed by Robson28 in 1969 as a modification of the system presented by Flocks and Kadesky in 1958.29 The American Joint Committee on Cancer’s (AJCC’s) latest definition of tumor-node-metastasis (TNM) classification (Table 43-4), provides more detailed information by separating characteristics of the primary lesion from those of regional nodes.30 These definitions can be used as either a clinical or a pathologic staging system. There is very poor correlation between the Robson and AJCC staging systems, as demonstrated in Table 43-5, making comparisons quite confusing.
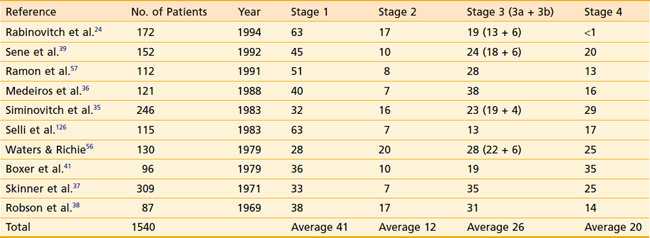
Distribution of patients by Robson stage at presentation is listed in Table 43-6. One must bear in mind that nearly all studies defining tumor stage at presentation are surgical series, resulting in a slight downward stage migration. It must be presumed that many patients with widespread disease at presentation (i.e., stage 4) are not surgical candidates and are therefore excluded from these reports. Overall, approximately 40% of patients have disease confined to the kidney, 12% have perinephric extension, 20% have renal vein extension, 5% have lymph node involvement, and 20% have extension to adjacent organs or distant disease.
TNM staging groups kidney-confined tumors as T1 or T2, and T3a represents involvement confined to the perinephric fat or adrenal gland. T3b and T3c lesions represent disease extending in the venous drainage, and T4 is locally advanced. N1 disease denotes only one regional lymph node, whereas N2 indicates involvement of at least two regional lymph nodes. TNM is also predictive of prognosis. Stage 1 and 2 patients have 5-year survivals of 74% to 96%.31
Prognostic Factors
A myriad of features, both clinical and pathologic, have been analyzed for the determination of their prognostic significance. The great majority of studies analyze individual factors by univariate analysis, ignoring the potential interdependence of factors on patient outcome. A thorough discussion of the relative importance of all such features is beyond the scope of this chapter; however, the interested reader is referred to an exhaustive review by Thrasher and Paulson.32
The most commonly evaluated prognostic factors relate to the gross anatomic extent of the primary lesion at the time of diagnosis; perinephric extension, renal vein extension, lymph node involvement, and metastatic disease have all been demonstrated by numerous authors33–38 to negatively affect patient survival. Pathologic features detectable by light microscopy have been evaluated, and several—tumor histologic type, grade, and cellular pattern—have repeatedly been found to have prognostic import.36,37,39 Patient features, such as age, sex, and race, and performance status have been considered relevant clinical prognostic factors by other authors.40–42
The Fuhrman nuclear grade is a well-established prognostic factor for RCC. Based on the shape and size of the nucleus, as well as the prominence of associated nucleoli, median 5-year disease-specific survivals of 94%, 86%, 59%, and 31% have been noted for grades 1 through 4 respectively.43
In terms of tumor histology, clear cell subtypes have the worst prognosis. However, in the metastatic setting, non–clear cell subtypes appear to do worse.44 Non–clear cell histologies are also more likely to involve lymph nodes.45
Microvascular invasion may also be an important factor in outcome. In a retrospective anlysis of 230 patients, those without evidence for microvascular invasion enjoyed a 5-year median disease-free survival of 87% compared with 27% for those with microvascular invasion (p < 0.001), and a disease-specific survival of 88% versus 40% (p < 0.001).46
Tumor size has also been noted to be an important prognosticator for RCC. When 11 cm was used to classify patients, those with larger lesions had a significantly worse outcome. Patients with larger lesions were more likely to develop DM disease, and 5-year disease-specific survival was 73% versus 57% (p < 0.001) in favor of smaller lesions.47
Molecular markers will likely play a more important role in assessing prognosis. Although data are yet to be validated in larger trials, reports have been published suggesting the utility of markers such as C-reactive protein, B7H1, B7H4, PTEN, P53, gelsolin, vimentin, and Ki67. In particular, P21 may play a role in the transformation of localized disease to metastatic.48,49
Rabinovitch and colleagues24 performed a multivariate analysis on a population of 172 surgically treated patients at Memorial Sloan-Kettering Cancer Center (MSKCC) and demonstrated that only renal vein extension (p = 0.001) and lymph node involvement (p = 0.026) were independent prognosticators for the development of DMs.24 Although positive margins and perinephric extension were considered significant on univariate analysis, these factors lost significance on multivariate analysis. Patient sex, radical versus PN, age, histologic type, and tumor size were not prognosticators on either univariate or multivariate analysis.
Once patients have distant disease, prognostic models can be useful for predicting survival. A commonly used prognostic nomogram was developed at MSKCC, in which Motzer and colleagues retrospectively reviewed the predictive factors for survival in 670 patients who had participated in 24 clinical trials from 1975 to 1996.50 Approximately 8% of patients had had prior cytokine therapy. Absence of nephrectomy, low hemoglobin, high serum-corrected calcium, high lactate dehydrogenase (LDH), and Karnofsky performance status lower than 80% were predictors of poorer survival. Furthermore, based on the number of risk factors, three risk groups were established: good or favorable risk, intermediate risk, and poor risk. If patients had no risk factors, they were a good or favorable risk and their median overall survival (OS) at 5 years was 22 months. If one to two risk factors were present, patients were of intermediate risk with a median OS of 10 months. But if a patient had three or more risk factors, they were deemed a poor risk and had a median OS of 4 months at 5 years. There was a significant difference in survival between the three risk groups (p < 0.0001). Several years later, the same group retrospectively analyzed 463 patients from clinical trials at MSKCC done from 1982 to 1996 who had been treated with interferon (IFN)-α and found that time from diagnosis to the need for initiation of IFN-α of less than 1 year was also a poor prognostic factor.51 The Cleveland Clinic later validated the MSKCC prognostic models in 353 previously untreated mRCC patients.52 They demonstrated that four of the five Motzer prognostic risk factors were independent predictors of survival (time from diagnosis to study entry, hemoglobin, corrected serum calcium, and serum LDH) and expanded the criteria to include prior radiotherapy (RT) (p < 0.001) and number of metastatic sites (more than one).
Surgical Management
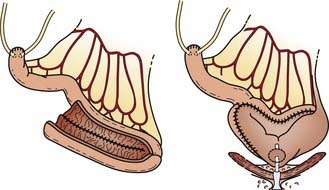
Radical Nephrectomy
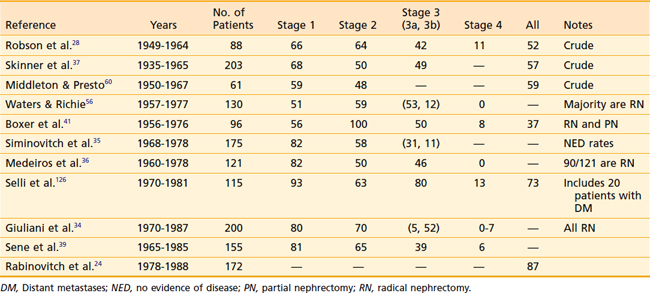
The RN defines the procedure in which the kidney, along with the Gerota fascia and its contents, are removed en bloc from the retroperitoneum. This procedure, first described by Mortensen38 in 1948, became the standard surgical technique beginning in the following decade, and is the standard of care today. The RN allows a more reliable margin around the known tumor than does a simple nephrectomy. Although the RN has never been compared with the simple nephrectomy in a randomized fashion, Robson in 1963 was the first to propose that the RN confers the best survival results.38
A summary of the results from surgical management of RCC is presented in Table 43-7. Despite the differences among published reports, the overall 5-year survival rates have changed little since Robson’s report in 1963. The relative constancy in outcome reflects not so much any factor regarding surgical management, but rather the lack of progress in any adjuvant treatment modality.