30 Cancer of the Hypopharynx
Epidemiology
Approximately 2600 cases of hypopharyngeal cancer are diagnosed in the United States each year, 75% to 80% of which arise in the pyriform sinuses and 15% to 20% from the posterior pharyngeal wall. Postcricoid cancers constitute about 5% of cases. The male-to-female ratio ranges from 5 : 1 to 7 : 1 for pyriform sinus cancer and 3 : 1 to 4 : 1 for pharyngeal wall cancer.1–3 Postcricoid cancers occur predominantly in women. The age-specific incidence rate for White men increases from the 40- to 44-year old group (0.4/100,000) to the 65- to 69-year-old group (9.6/100,000) and then subsequently declines (4.3/100,000) for the 85-year-old group.2 Among African-American men, the incidence continues to increase with age (up to 15.8/100,000 in the 85-year-old group).2 In general, the prognoses for hypopharyngeal tumors are notoriously poor, because of the following associated factors: extensive local-regional spread of disease through the submucosal route, abundant lymphatic drainage of the region with a greater propensity for distant metastatic spread; clinical presentation at an advanced stage; frequent association with nutritional depletion; concurrent medical problems conferred by tobacco and alcohol abuse; and occurrence in patients with a predisposition for the development of second malignancies (secondary to mucosal field cancerization).
Etiology
Tobacco and alcohol use are clearly associated with the development of hypopharyngeal cancer.4–6 Although tobacco alone is known to be carcinogenic, the role of alcohol in the etiology of hypopharyngeal cancer has been underappreciated. A prevailing opinion is that alcohol potentiates carcinogenesis in tobacco-exposed mucosa.7 However, evidence points to an independent role for alcohol.5,8–10 In never smokers, an independent association of heavy alcohol consumption and oropharynx/hypopharynx/larynx cancer was found in a recent pooled analysis by the International Head and Neck Cancer Epidemiology Consortium.10 Other data suggest a synergistic interaction between tobacco and alcohol with regard to the risk of inducing hypopharyngeal cancer, particularly for people with heavy alcohol consumption.5,9,11 Concurrent alcohol consumption and smoking has been found to be associated with a multiplicative increase in risk to 10 to 20 times higher than nonsmokers/nondrinkers.12,13 The risk for hypopharyngeal and oropharyngeal cancer may be especially high in patients with inactive or less active forms of the ADH1B and ALDH2 enzymes.14
Cigarette smoking is clearly a strong risk factor for head and neck cancer independent of alcohol drinking, with a relative risk of 4.0 to 5.0 for oropharyngeal and hypopharyngeal cancers.10 Among cigarette smokers, the use of unfiltered cigarettes or black tobacco (with higher concentrations of carcinogenic N-nitrosamines) increases the risk of cancer.5 For a similar reason, smoking marijuana (with its high tar burden and aromatic hydrocarbon content) appears to confer even greater risk.7 Moist snuff (smokeless tobacco) consumption also exposes the pharynx to carcinogens.15,16 The latter is noteworthy in that snuff consumption increased by 38% in the United States between 1981 and 1993, paralleled by a three-fold increase in snuff sales between 1980 and 2003.17,18 A growing prevalence of snuff dipping among young men, non-Hispanic Whites, people with lower educational attainment, and residents of Southern U.S. states and rural areas has been suggested as a reason for this increase.17,19 Dietary factors predisposing to the development of hypopharyngeal cancer may include a diet low in carotenoids4,7 and iron-deficiency anemia. This anemia has been associated with postcricoid cancers in women in Scandinavia and Great Britain and usually occurs in this setting along with dysphagia (from hypopharyngeal webs) and atrophy of the oral mucosa (manifested by loss of tongue papillae). The triad of iron-deficiency anemia, dysphagia, and glossitis is known as the Plummer-Vinson syndrome in Scandinavia and the Paterson-Brown Kelly syndrome in Great Britain.
Genetic susceptibility of the host influences the likelihood of cancer development.4 DNA repair capability, which can be indirectly measured by assessment of chromosomal breakage induced by in vitro exposure to bleomycin,20 was demonstrated in a case–control study to be a strong predictor of the risk of upper aerodigestive tract cancer.21 However, whereas bleomycin-induced mutagen sensitivity was higher in patients with hypopharynx cancer compared to smoking controls, there was no association between mutagen sensitivity and the risk of squamous cell carcinoma of the hypopharynx. Mutagen sensitivity and its association with cancer risk appeared to decrease from the oral cavity towards the oropharynx and larynx.22 A positive family history of cancer of the respiratory or upper digestive tract among first-degree relatives (especially siblings) also has been associated with an increased risk of head and neck cancer.23
Genetics and Cytogenetics
Genetic abnormalities that appear to be causally linked to the etiology of head and neck cancer are point mutations in the p53 gene24 and bcl-2 overexpression. In the case of bcl-2, the protein encoded by the bcl-2 gene is overexpressed in head and neck cancers among heavy smokers.25 This is hypothesized to result from the chromosomal translocation t(14;18)(q32;q21), which juxtaposes the bcl-2 proto-oncogene with the immunoglobulin heavy-chain gene,26 a juxtaposition that occurs in 85% of non-Hodgkin’s follicular lymphomas.27 It is further hypothesized that carcinogens from cigarette smoke act upon the bcl-2 site and increase the rate of the t(14;18) translocation.25,26
Other oncogenes may be involved in the pathogenesis of head and neck cancer; these include p16 (CDKN2), PRAD1 (cyclin D1), EMS1, the epidermal growth factor (EGF) receptor gene, erb-B-2 (neu), myc, and ras.7,24,28–31 Particular oncogenes are expected to act at distinct points along a multistep pathway in a manner analogous to the colorectal model demonstrated by Vogelstein and associates.31,32
Amplification of the 11q13 region, which encodes for cyclin D1, FGF3, FGF4 and EMS1, has been found to be the most common genetic alteration in hypopharynx carcinomas.33 Cyclin D1 polymorphism has been shown to be associated with poor differentiation and reduced disease-free interval in pharyngeal and laryngeal squamous cell carcinomas.34,35 FGF3 and FGF4 amplification has been correlated with nodal disease, and myc amplification with increasing T-stage. Increased NBS1 expression has been correlated with poor prognosis and decreased overall survival in advanced head and neck squamous cell carcinoma and has been suggested to activate the PI3/Akt pathway and phosphorylation of its downstream target mTOR.36 More recently, hypermethylation has been suggested to represent an important epigenetic alteration in head and neck cancer. One study showed hypermethylation of p16, MGMT, DAP-K, and E-cadherin to be between 27% and 44% in a large group of hypopharynx and larynx cancers. Attempts to correlate hypermethylation with survival have failed to date.37
As a vector of proto-oncogenes, human papillomavirus (HPV) infection may also contribute to the etiology of hypopharyngeal cancer.38 Integration of its DNA into the host genome and expression of early viral proteins, E6 and E7, affects host cellular functions by induction of hyperproliferation, inhibition of apoptosis, altered transcriptional activity and disruption of cell-to-cell interactions.13 HPV-E6 and E7 proteins inactivate p53 and pRb pathways, thereby upregulating p16.39 By contrast, loss of p16 and TP53 gene mutation appear to be early events in tobacco- and alcohol-related squamous cell carcinomas.39 Oral HPV16 infection has been found to be associated with oropharyngeal cancers in patients with and without a strong history of tobacco and alcohol use.40 One study has associated detection of human papillomavirus DNA in laryngeal and hypopharyngeal tumor specimens with both decreased disease-specific survival and decreased local-regional control.38 However, most studies have shown that HPV-associated squamous cell carcinoma carries a favorable prognosis compared to HPV-negative tumors. A favorable prognosis subgroup can be identified using HPV-16 viral load and p16 expression.41,42 Not all studies have detected HPV16 in non-oropharyngeal head and neck subsites.42,43
Anatomy
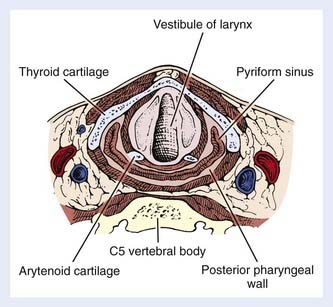
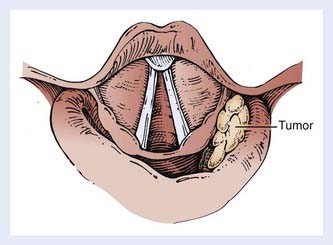
The hypopharynx (the inferior portion of the pharynx) is subdivided into three sites: (1) the pyriform sinuses (the paired recesses lateral to the larynx), (2) the posterior pharyngeal wall, and (3) the postcricoid region. The hypopharynx functions to guide food into the esophagus and away from the larynx, during normal swallowing. The hypopharynx extends from the level of the hyoid bone to the inferior edge of the cricoid cartilage, corresponding to the C4 through C6 vertebral bodies. It envelops the larynx, as shown in Fig. 30-1. The oropharynx lies above, the cervical esophagus below, the larynx anteriorly and medially, and the retropharyngeal space posteriorly.
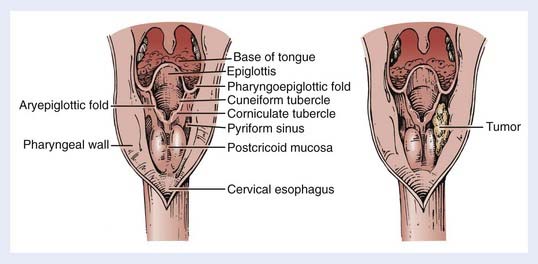
The bilateral pyriform sinuses (laryngopharyngeal sulci) lie just distal to the glossopharyngeal sulci of the oropharynx. The pharyngoepiglottic fold demarcates each pyriform sinus from the oropharynx (Fig. 30-2). Underlying and inferior to this fold is the pharyngoepiglottic muscle, which constitutes the anterior wall of the pyriform fossa. The medial wall is composed of the mucosal surface overlying the aryepiglottic muscle (lateral to the aryepiglottic fold), as well as the surface lateral to the arytenoid cartilage. Lateral boundaries are the thyrohyoid membrane (upper portion) and thyroid cartilage (lower portion). Inferiorly, the sinus tapers to an apex, the level of which varies among individuals but usually is no lower than the lower border of the cricoid cartilage. The apex is composed of loose, expansile connective tissue. Posteriorly, the pyriform sinus opens to the remainder of the hypopharyngeal lumen.
The posterior pharyngeal wall is continuous with the lateral walls of the two pyriform sinuses (see Fig. 30-1). The wall itself, from the inner to the outer aspect, is composed of the following: mucosal lining, submucosa, fibrous layer (pharyngobasilar fascia), muscular layer, and a deeper layer of fascia (visceral fascia). The muscular layer is composed mainly of the inferior constrictor muscle, consisting of the thyropharyngeal, cricopharyngeal and tracheopharyngeal muscle inferiorly, with contributions from the distal portion of the middle constrictor muscle superiorly. They insert in the pharyngeal raphe posteriorly. These muscles contract in the last phase of deglutition, thus, increasing the pressure on the food and passing it on to the peristaltically-moving esophageal muscles. Behind the posterior pharyngeal wall, loose connective tissue forms a potential space (the retropharyngeal space) between this wall and the prevertebral fascia. Posterolaterally lie the carotid spaces, which contain the neurovascular bundles; these are intimately associated with the deep cervical lymph nodes and vessels.
The postcricoid area is the caudally sloping mucosal surface that overlies the posterior lamina of the cricoid cartilage (see Fig. 30-2). The posterior surfaces of the arytenoid cartilages form the superior boundary of this region. The posterior cricoarytenoid muscles lie immediately underneath the mucosa. Inferiorly, the postcricoid area is continuous with the cervical esophagus.
The hypopharynx receives its arterial supply from pharyngeal branches of the superior and inferior thyroid arteries. Venous drainage occurs through the pharyngeal plexus, posterior to the pharyngeal constrictor muscles. Sensory nerve supply to the hypopharynx is provided through both the internal branch of the superior laryngeal nerve and the recurrent laryngeal nerve. Motor supply is predominantly via the recurrent laryngeal nerve. Both are branches of the vagus nerve (cranial nerve X). The internal branch of the superior laryngeal nerve runs submucosally along the anterolateral wall of the pyriform sinus and exits the laryngeal framework superolaterally through an opening in the thyrohyoid membrane. This branch eventually joins the vagus nerve, which synapses with the auricular nerve of Arnold at the jugular ganglion; this pathway allows for referred otalgia (see Clinical Presentation). Branches of the recurrent laryngeal nerve course submucosally through both the postcricoid region and the pyriform fossa.
Lymphatic Drainage
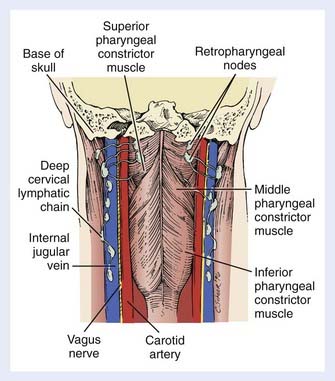
The retropharyngeal lymph nodes can also be accessed from the hypopharynx. These nodes are located high in the retropharyngeal space and are divided into a medial group and a lateral group (Fig. 30-3) (the latter are also called the nodes of Rouvière). Both groups are above the level of the hyoid bone. Cephalad spread results in retropharyngeal nodal involvement up to the base of skull. Below the hyoid, the retropharyngeal space contains fat only. Yet, as there are no fascial barriers within this space, once tumor penetrates it, neoplastic cells can migrate freely throughout. Caudal dissemination allows disease to access the mediastinum, with which the retropharyngeal space is contiguous. This space thus becomes an additional vertical pathway through which tumor cells may travel and develop skip metastases.
Pathology
Squamous cell carcinomas constitute more than 95% of carcinomas of the hypopharynx. Among pyriform sinus carcinomas, approximately one third have been found to be nonkeratinizing and 40% poorly differentiated in one large series.44 The same report noted the tumor growth pattern at the periphery of biopsy specimens to be infiltrating in 80% and pushing in 20%.44 Trends toward increased local-regional relapse were found for keratinizing (vs nonkeratinizing) tumors and for those with infiltrating (vs pushing) margins.44
This information has greater relevance in light of reports from the Department of Veterans Affairs Cooperative Laryngeal Cancer Study Group that demonstrate that the histologic growth pattern of the tumor (for cancer of the larynx) is a significant predictor of both complete tumor response to neoadjuvant chemotherapy45 and disease-free survival.46 Cancers with blunt, thick invading cords of tumor responded less frequently—and were associated with a poorer disease-free survival rate—than those with thin, irregular infiltrating cords. The latter pattern is thought to reflect rapidly growing tumor with a high proliferative rate.45
The basaloid variant of squamous cell carcinoma occasionally arises in the hypopharynx and is associated with an aggressive biologic behavior and poor prognosis.47–49 Other rare tumors of the hypopharynx include minor salivary gland tumors, sarcomas of various types (fibrosarcomas, liposarcomas, synovial cell sarcomas, and malignant fibrous histiocytomas), and lymphomas.
Clinical Presentation
Symptoms
The most common presenting symptoms of cancer of the hypopharynx are sore throat, odynophagia, and a neck mass. As primary hypopharynx tumors remain asymptomatic for a long time and typically present at an advanced stage, a neck mass may be the sole presenting symptom in up to 70% of cases.50–54 Dysphagia and weight loss frequently occur with locally advanced disease but can occur in early stages as well. In very advanced stages, airway obstruction requiring emergency tracheostomy occurs in 5% to 10% of cases. Voice changes may develop in tumors with involvement of the larynx or the recurrent laryngeal nerves. Other symptoms of locally advanced lesions may include otalgia, neck pain, foreign body sensation, mucus retention in the throat, aspiration, and hemoptysis.
The hallmark of postcricoid carcinoma is dysphagia, which is a presenting symptom in more than 90% of cases.55–57 Among posterior wall cancers, dysphagia heralds the disease in 45% to 90% of patients.51,58–60 Other comorbidities may include malnutrition due to alcohol abuse, alcohol-induced liver disease, and chronic obstructive pulmonary disease.
Physical Examination
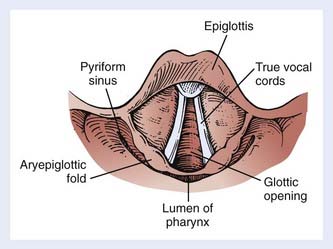
Indirect mirror and direct fiber-optic endoscopic examination of the laryngopharynx are mandatory for evaluation of disease. Schematic endoscopic views of the normal and diseased hypopharynx are shown in Figs. 30-4 and 30-5. Pooling of secretions and arytenoid edema may be prominent findings, especially with tumors of the pyriform apex and postcricoid region. It is not unusual for these findings to obscure visualization of the primary lesion itself. Pyriform sinus or postcricoid lesions usually appear as flat plaques with raised edges and superficial ulceration.52 Tumors of the upper (membranous) portion of the pyriform sinus tend to be locally infiltrating, exophytic lesions, whereas those of the lower (cartilaginous) portion tend to be widely infiltrating, ulcerative lesions. Lesions arising from the cartilaginous portions of the hypopharynx have been associated with a poorer prognosis compared with lesions from the membranous portion of the pyriform region,61–63 and so it is important to note from which portion the tumor arises. It is also important to differentiate a lesion of the medial wall of the pyriform fossa from a lesion arising from the aryepiglottic fold proper, as the latter carries a better prognosis.64 Vocal cord symmetry and mobility should be evaluated with and without phonation in order to rule out endolaryngeal invasion. Posterior wall tumors can present as submucosal bulges with or without ulceration. They tend to be exophytic and are often large (80% >5 cm) at presentation and may extend to the posterior oropharyngeal wall superiorly.52
Routes of Disease Spread
Local
Noteworthy routes of local spread include submucosal extension of disease, not infrequently associated with skip metastases and intervening normal-appearing mucosa.65 Such spread allows for both vertical and horizontal migration of the tumor. Medial extension results in involvement of endolaryngeal structures. The primary tumor can also extend directly into the soft tissues of the neck through the thyrohyoid membrane, through the thyroid cartilage, or around the posterior border of the thyroid ala. In the latter two instances, the thyroid gland may also be invaded, which is a poor prognostic factor.52
Tumors arising in the pyriform apex often invade the thyroid cartilage.66 Tumors originating from the medial wall or the angle of the pyriform sinus commonly invade the paraglottic space, laryngeal cartilages, the apex of the pyriform sinus, and the esophageal verge. Vocal cord fixation may result from involvement of the cricoarytenoid joint, invasion of the posterior cricothyroid muscle, or involvement of the recurrent laryngeal nerve.66–68 Superiorly, pyriform sinus carcinomas commonly extend to the lateral wall of the oropharynx and the base of the tongue.52 Posterior pharyngeal wall carcinomas frequently extend to the oropharynx superiorly52 and appear as an asymmetrical thickening of the posterior pharyngeal wall on imaging.69 Postcricoid tumors commonly spread submucosally toward the cervical esophagus and trachea.69 They also tend to grow anteriorly to involve the posterior cricoarytenoid muscle and may extend through the cricoid cartilage into the trachea.52
Invasion of the carotid artery is suggested when tumor encircles the artery on 3 sides, or 270 degrees or more. Focal stenosis of the carotid artery segment surrounded by tumor also suggests invasion. Invasion of the trachea should be carefully assessed.70,71 Local control of T1-T2 pyriform sinus tumors treated with definitive radiation therapy (RT) has been found to be related to tumor volume more than 6.5 mL and bulky apex disease ≥10 mm.72
Nodal
Nodal involvement is a poor prognostic factor and occurs more commonly in hypopharyngeal cancer than in other head and neck subsites.71,73 Hypopharynx carcinomas most commonly spread to level II nodes, with level III and IV lymph nodes particularly being involved in clinically node-positive patients.52,74 Eleven percent have level V lymph node involvement.74 For pyriform sinus cancers, palpable lymph nodes can be found at presentation in 70% to 75% of patients.53,75–77 Bilateral or contralateral nodes are present in 10% to 14%.75,76,78 Neck dissection increases the yield of node positivity by exposing occult disease. Upon dissection, up to 40% to 50% of patients with clinically N0 necks can be found to harbor nodal metastases.79–81
Posterior wall tumors present with palpable nodes in 40% to 60% of cases.60,82–85 The incidence of bilaterality ranges from 10% to 35%,82,83,85 depending on the location of the primary tumor in the medial-to-lateral dimension. The incidence is higher with more medially located tumors, due to submucosal anastomoses of lymphatic capillaries. Occult metastases have been found in 54% to 66% of N0 patients upon neck dissection.51,86 Between 30% and 40% of patients have retropharyngeal lymph nodes involved with tumor.82,87,88
Postcricoid cancers present with palpable nodes in 33% to 45% of patients.55–57,89–91 Bilateral or contralateral nodes are present in 8% to 18%.56,91 Among patients with T4 tumors, 50% have clinically apparent nodal disease, and 33% have bilateral involvement.57 Occult metastases may frequently be present in paratracheal lymph nodes or the thyroid gland (which is also true for tumors originating in the adjacent apex of the pyriform sinus).86,92–95 Such subclinical disease must be taken into account when the treatment is planned.96–99
Distant
Of patients with hypopharyngeal cancer, 20% to 30% develop clinically overt distant metastases within 2 years, despite treatment. The autopsy incidence of distant metastases among 108 patients treated in the modern era at a single institution was found to be 60%. However, only 13% of these patients had pathologic local-regional control of disease.100 Autopsy studies reporting on heterogeneous cases of head and neck cancer have shown a 19% to 42% incidence of distant metastases among patients who have no evidence of local-regional disease at postmortem examination.101 This suggests the presence of micrometastatic spread of disease at the time of initial diagnosis. Results from a Radiation Therapy Oncology Group (RTOG) analysis of the effect of local-regional control on distant metastatic dissemination for 237 cancers of the hypopharynx support this hypothesis: local-regional control of disease at 6 months was associated with a slight reduction in the incidence of distant metastases at 0.5 to 2.5 years, from 43% to 40% (P = .054).102
The most common metastatic sites, in decreasing order, are lung, mediastinal nodes, liver, and bone.100
Diagnostic and Staging Studies
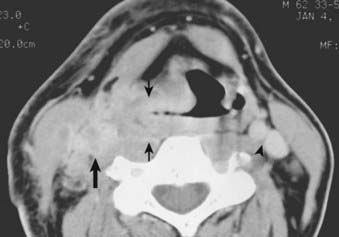
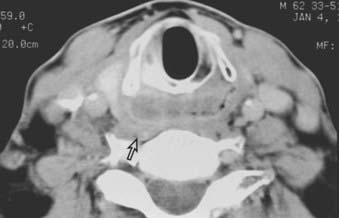
Routine staging studies should include a chest x-ray, blood tests to evaluate liver function, and a computed tomographic (CT) scan with intravenous contrast of the head and neck. The CT scan should be done before biopsy of the tumor to avoid imaging postbiopsy edema, which obscures radiologic definition of tumor extent.103 The CT scan is useful for precise delineation of tumor extent, with particular reference to the following: visualizing inferior extent of tumor (for which fiber-optic endoscopy alone is often inadequate); detecting invasion of the laryngeal framework and extralaryngeal/extrapharyngeal spread into the soft tissues of the neck; detecting submucosal spread toward the midline via the paraglottic space (anteromedial to the pyriform sinus); and detecting posterior spread into the retropharyngeal space (Figs. 30-6, 30-7, and 30-8).104 It is also useful for disclosing clinically occult metastatic lymphadenopathy, particularly positive retropharyngeal nodes, contralateral nodes, and extracapsular extension of disease. Limitations of CT imaging are failure to detect small superficial tumors, overestimating the tumor extent due to inflammatory and edematous changes, early laryngeal cartilage involvement, and distortion of adjacent normal structures that may mimic tumor involvement.71
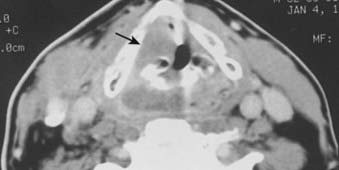
FIGURE 30-7 • Pyriform sinus cancer extending into paraglottic space. Endoscopically, mass effect upon the laryngeal vestibule was apparent.
Helical (spiral) CT provides a rapid and practical means for three-dimensional (3D) imaging of the upper airway.105 It can be used to produce a 3D airway cast—similar to the laryngogram done in years past (but not requiring instillation of foreign material). In demonstrating tumor mass effect upon adjacent airway structures clearly, the 3D laryngogram provides a complementary view of tumor extent.
Magnetic resonance imaging (MRI) is an important adjunctive study in three situations: (1) for determining whether cartilage invasion is present, which is shown by an increase in T2 signal intensity, a low to intermediate signal on T1-weighted images, and postcontrast enhancement69,106,107; (2) for determining the extent of paraglottic space invasion; and (3) for determining esophageal invasion, which is characterized by an increased T2 signal intensity, wall thickening, and effaced fat planes.106,108,109 These are pertinent issues when conservation laryngopharyngeal surgery is being considered, because cartilage invasion and extensive paraglottic space invasion are contraindications to such surgery. MRI is helpful in ruling out esophageal involvement.109 It shows better soft tissue contrast than CT scan and often less artifact from dental fillings than CT scan.71 However, because of the relatively long imaging time, the advantages of soft tissue detail may be outweighed by motion artifacts, especially at the level of the larynx and hypopharynx.106 Newer MRI techniques, including diffusion-weighted imaging, MR spectroscopy, and supermagnetic iron oxide contrast agents, may allow clearer distinction between benign and malignant lymph node involvement, sites of hypoxia, or identification of edema and necrosis.71,110–112
Positron-emission tomography (PET) has seen a rapid rise. It has a detection rate for head and neck primary tumors between 88% and 98%113–115 and, in general, has a higher sensitivity than either CT or MRI.115,116 Limitations include failure to detect very small primary tumors, superficial lesions, and locations near tissues with physiological intense 18-Fluoro-2-deoxy-D-glucose (FDG)-uptake like salivary tissues, Waldeyer’s ring, muscles, and larynx in patients who speak during the FDG uptake phase.117 In evaluating nodal involvement, FDG-PET has a higher sensitivity and specificity than CT, MRI, or ultrasound.118–120 PET/CT as a combined modality appears to be better than PET+CT or CT alone.106,119 However, in clinically N0 necks, despite an approximate incidence of 25% to 50% of occult metastases, PET has failed to show acceptable sensitivity or specificity.121,122 This may in part be related to the spatial resolution of 4 to 5 mm for PET scans.121 PET imaging has been found to alter the TNM score in 15% to 36% of patients compared to CT- or MRI-based staging.123–126 Because of the greater sensitivity for the detection of metastatic lesions and the ability to image the whole body, PET/CT may be used for staging and detection of synchronous second primaries in appropriately selected head and neck patients at >10% risk for distant metastases.116,127–129 These include patients with ≥4 lymph node metastases, level 4, bilateral, or ≥6 cm lymph node metastases, recurrent tumors, or presence of a second primary tumor.130 However, concerns about a false positive rate of up to 20% in some studies131,132 and the relatively small number of patients in available studies to date, require any PET findings to be corroborated by pathologic confirmation.117,133
Biopsy of the primary lesion should be performed under general anesthesia. Because of the risk of synchronous primary malignancies amid vulnerable mucosa, bronchoscopy and esophagoscopy should also be done at this time.134,135 Open biopsy of a neck mass to obtain a tissue diagnosis should be avoided, as this oncologically contaminates the neck.
Staging
The 2010 American Joint Committee on Cancer (AJCC) staging136 for all hypopharyngeal cancers is given in Table 30-1. Note that the staging for T2 lesions maintains the size parameter as well as the proviso that there is no fixation of the hemilarynx. Stage T4a includes invasion of the thyroid gland, cricoid and thyroid cartilage hyoid bone, or central compartment of soft tissue, which includes prelaryngeal strap muscles and subcutaneous fat. Please note that invasion of the esophagus is no longer considered stage T4a, but T3. Stage T4b includes invasion of the prevertebral fascia, encasement of the carotid artery, or invasion of the mediastinal structures.
Standard Therapeutic Approaches
Pyriform Sinus: Early-Stage (T1-T2) Disease
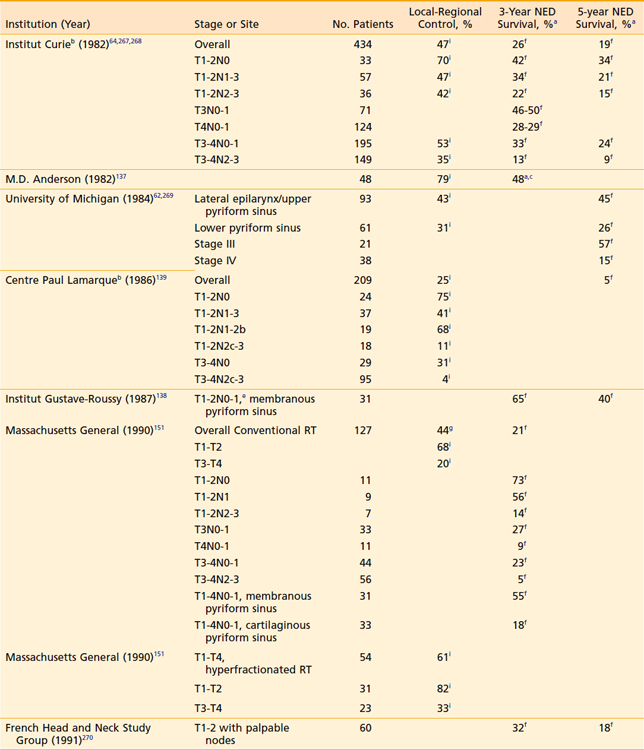
Early-stage favorable disease is not commonly encountered. In each of three separate series of more than 400 patients, T1 and T2 tumors (endophytic included) with nonfixed nodes constituted 12% to 16% of the cases; categorizing disease by the additional features listed previously would further winnow the number of patients actually presenting with favorable disease.64,137–139 Earlier detection of hypopharyngeal cancer, however, may increase the fraction of patients who are eligible for a larynx-conserving approach.
Either a primary conservative surgical approach or primary radiation therapy is a suitable treatment option for early-stage pyriform sinus cancer. Features allowing for conservative therapy are the following: all T1 tumors as well as T2 exophytic tumors of the upper or membranous portion of the pyriform sinus, lack of extension to the apex, lack of disease extension to the arytenoids, intact vocal cord mobility, uncompromised airway, and nonfixed neck nodes.62,63,140–151 Tumors originating in or extending to the apex frequently invade the thyroid cartilage, which often necessitates its removal in surgical management of disease respecting oncologic principles.66 Furthermore, in obtaining clear surgical margins on apical disease, the cricoid cartilage often must be sacrificed, necessitating permanent tracheostomy. In addition, patients require adequate pulmonary function to undergo a conservative hypopharyngeal surgery.
Conservative surgery consists of partial laryngopharyngectomy and ipsilateral modified radical neck dissection (or bilateral for N2c disease).150,152–157 In the setting of an N0 or N1 neck, a lateral neck dissection (involving removal of level II, III, and IV nodes) may be performed instead. Partial laryngopharyngectomy removes the upper portion of the pyriform sinus and adjacent marginal laryngeal structures (false cords, epiglottis, and possibly the ipsilateral arytenoid cartilage). In the setting of positive margins, multiple positive lymph nodes, or extracapsular extension of disease, surgery is followed by postoperative radiotherapy to maximize locoregional control of disease.76,78,80,92,158–160
When primary radiation therapy is decided upon, a planned neck dissection may follow radiotherapy, approximately 3 to 4 weeks after completion of radiotherapy. This approach may be performed if any single lymph node is greater than 2 or 3 cm or if multiple positive lymph nodes are present on initial clinical examination, unless there has been early complete regression of neck disease.63,64,142,149,161,162 A selective or radical neck dissection may be performed for persistent nodal disease after a course of definitive radiation therapy.163,164
Amdur et al165 reported on 101 patients treated at the University of Florida with radiotherapy alone with or without a planned neck dissection. The 5-year actuarial local control rates for T1 and T2 tumors were 90% and 80%, respectively. After surgical salvage, the ultimate local control was 95% and 91%, respectively. On univariate analysis apical involvement for T1 tumors was associated with an inferior local control compared to patients with no apical involvement (1/3 [33%] versus 14/14 [100%]).
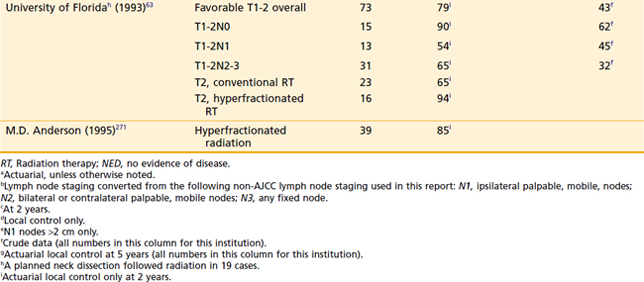
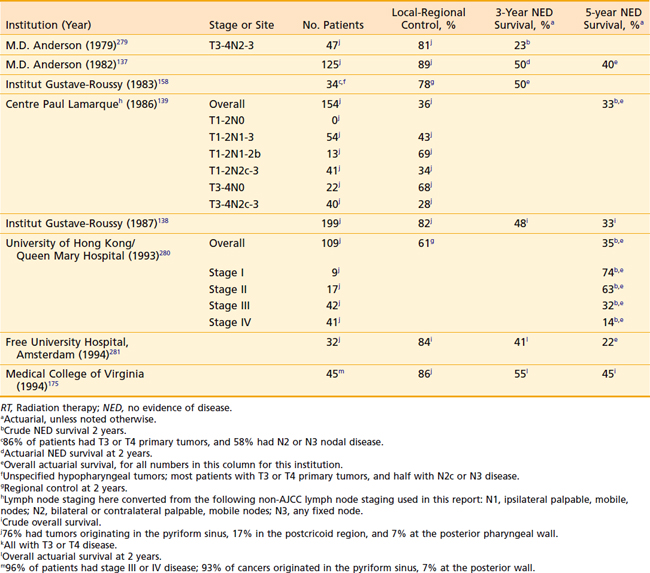
Although the local-regional control and survival outcomes are similar for conservative surgery and radiotherapy (Tables 30-2 and 30-3),142,144,166,167 primary radiation therapy may be the preferable treatment option because of its associated superior functional outcomes involving speech and swallowing. Nevertheless, there is an increased risk of swallowing dysfunction after radiotherapy for early-stage lesions. In 101 patients in the University of Florida series, 6% of patients experienced difficulty swallowing, requiring a permanent gastrostomy tube after radiotherapy alone.165 Although, as noted, there are potential significant complications after radiotherapy alone, the risk of severe toxicity and significant dysfunction is more prevalent after conservation surgery. Furthermore, the risk of chronic aspiration secondary to pharyngolaryngeal dyssynergia (due to sacrifice of the superior laryngeal nerve) after conservation surgery is less often observed after definitive radiation therapy.143,155,156,168,169
Pyriform Sinus: Locally Advanced (T3-T4) Resectable Disease
Postoperative radiation therapy is preferred to preoperative irradiation. A randomized trial by the RTOG, RTOG 73-03, has been the definitive study of this sequencing issue.170,171 Postoperative irradiation was associated with significantly improved local-regional control for all anatomic subsites combined. Among hypopharyngeal cases only, actuarial local-regional control and survival at 4 years were 61% and 28%, respectively, for the postoperative group, compared with 50% and 18% for the preoperative group. Although other studies have suggested an increased perioperative complication rate in particular in patients with hypopharyngeal cancer who are treated with preoperative irradiation,50,147,172,173 this relationship was not confirmed in RTOG 73-03.
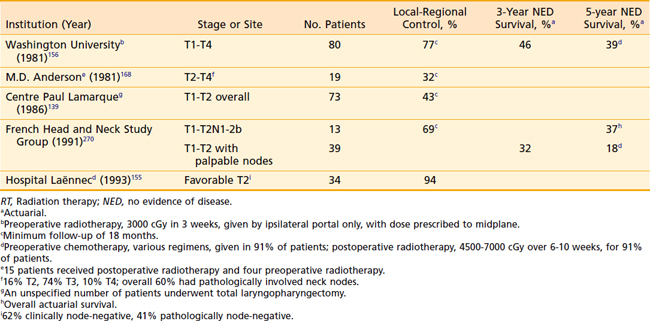
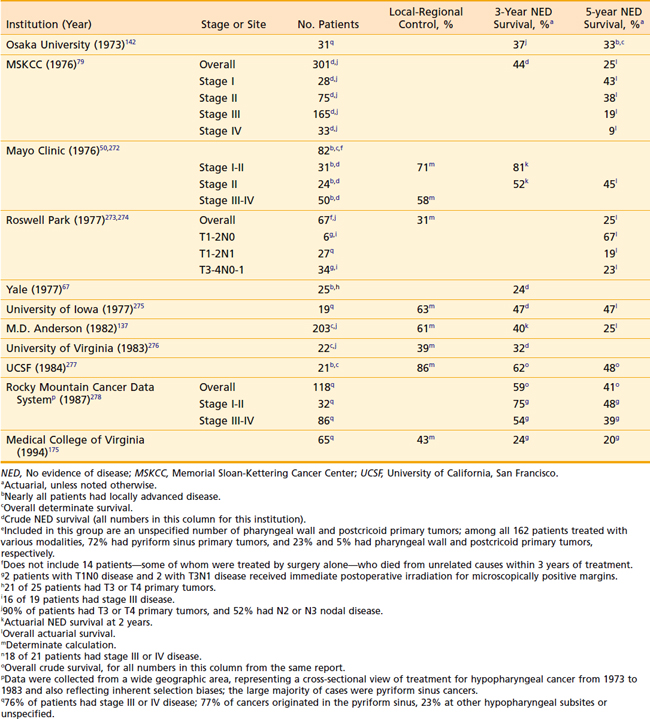
For stage III and IV disease, surgery in combination with postoperative radiation therapy results in better local-regional control rates (and possibly survivorship) than surgery alone. Retrospective data supporting this conclusion are outlined in Tables 30-4 and 30-5. The only randomized trial that compared surgery alone with surgery plus postoperative radiation therapy in the management of locally advanced head and neck cancer, including pharyngeal carcinomas, did not adequately address the question.174 The case mixture was heterogeneous, the sample size small, and the distribution of prognostic factors between groups unequal despite randomization.175 Furthermore, the total dose of radiation administered (50 Gy) was too low to eradicate microscopic disease in an operative bed.
Because distant metastases remain a common reason for treatment failure despite adequate local-regional control of disease,102,138,174–176 systemic therapy is an important consideration in the management of patients with stage III or IV disease. In randomized trials involving heterogeneous cases of advanced head and neck cancers, induction chemotherapy has been shown to affect the incidence of distant metastases.177–179 Adjuvant chemotherapy both after definitive local-regional therapy (as maintenance chemotherapy)180,181 and concurrent with local therapy (in the sequence surgery-chemotherapy-radiation therapy)182 may also reduce the rate of distant failure. Similarly, chemotherapy as part of a larynx-preserving treatment approach may result in decreased failure below the clavicles, as it has been shown in the management of advanced laryngeal cancer.183
Larynx-conserving treatment regimens in which aggressive surgery is avoided have become increasingly common.184–200 Early results from a randomized trial led by the European Organization for Research in Cancer Therapy comparing induction chemotherapy (three cycles of cisplatin and 5-fluorouracil [5-FU]) and radiotherapy with primary surgery and postoperative radiotherapy suggested that larynx preservation might be a viable strategy in managing hypopharyngeal cancer, as survival was not compromised.184 At 3 years, the crude survival rates were 53% for the chemotherapy-radiotherapy approach and 56% for the surgery plus postoperative radiotherapy arm, respectively, and 28% of patients in the chemotherapy-radiotherapy arm were free of disease, with an intact, functioning larynx. Follow-up data from this trial confirm these positive results: Survival rates in the two arms remained equivalent, whereas in the experimental arm, local progression-free survival with a functional larynx was achieved in 35% of patients at 5 years.185
An Intergroup trial (RTOG 95-01, ECOG R9501, SWOG 9515) showed that concomitant postoperative chemotherapy and radiation improves local-regional control and disease-free survival in a high-risk group of squamous cell carcinoma of the head and neck.201 Patients were randomized to postoperative radiotherapy alone (60 to 66 Gy in 30 to 33 fractions) versus concurrent radiotherapy and cisplatin 100 mg/m2 on days 1, 22, and 43. Ten percent of all patients had a primary hypopharyngeal tumor. The high-risk group was defined as: two or more regional lymph nodes involved on histology, extracapsular extension of nodal disease, and microscopically involved mucosal margins of resection. After a median follow-up of 45.9 months, 30% local-regional recurrences were observed with radiotherapy alone versus 19% with the use of concurrent chemotherapy. The two-year rate of local-regional control was 72% for radiotherapy alone and 82% for combined therapy. Disease-free survival was significantly longer after concurrent chemotherapy and radiation compared to radiotherapy alone (hazard ratio 0.78). There was a trend towards improved overall survival with concurrent chemotherapy and radiation. However, the incidence of acute adverse effects of grade 3 or greater substantially increased from 34% with radiation alone to 77% of patients receiving combined therapy.
The EORTC performed a similar trial for stage III or IV head and neck cancer comparing adjuvant concomitant cisplatin 100 mg/m2 (days 1, 22, and 43) and irradiation with radiotherapy alone (66 Gy in 33 fractions in both arms).202 Twenty percent of patients had a hypopharyngeal primary tumor site. At 5 years, local-regional control (31% versus 18%), progression-free survival (47% versus 36%) and overall survival (53% versus 40%) were significantly higher in the combined therapy group. Although there were more severe grade 3 or higher acute adverse effects in the combined therapy group (41% versus 21%), the incidence of severe mucosal and late adverse effects was similar in both groups.
Given the high toxicity of adjuvant chemoradiation, it is recommended only for high-risk, physically fit patients. In a retrospective subgroup analysis of both trials, chemoradiation improved the outcome most significantly in patients that had extracapsular extension and/or microscopically involved surgical margins.203
Pyriform Sinus: Unfavorable, Unresectable Disease
Concomitant chemotherapy and radiation therapy is rapidly becoming the standard management for locally advanced, unresectable head and neck cancer, including hypopharyngeal cancer.200,204–209 Randomized trials involving heterogeneous groups of unresectable, epithelial head and neck cancers have demonstrated the survival benefit of this approach compared with induction chemotherapy with radiotherapy, as well as the use of hyperfractionated radiotherapy regimens without chemotherapy.210,211 Although cisplatin often exacerbates the mucositis caused by radiotherapy, it is the chemotherapeutic agent most commonly used in the concomitant setting, as it is associated with high response rates.212–214 In most series, mucositis is observed in approximately 70% to 80% of treated patients and pretreatment placement of a percutaneous gastrostomy is recommended. Chemotherapy typically is given on day 1, 22, and 43 of a radiotherapy treatment schedule (see Techniques of Radiotherapy).212,213,215
Intensity-modulated radiation therapy with concurrent chemotherapy continues to replace older radiation techniques. Lee et al. reported a 2-year locoregional progression-free survival of 73% and 2-year overall survival rates of 53% for stage III and IV hypopharyngeal cancer.247 Studer et al reported 2-year local control and overall survival rates of 90%.216
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree