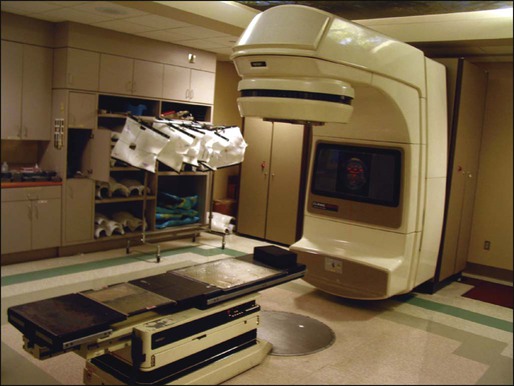
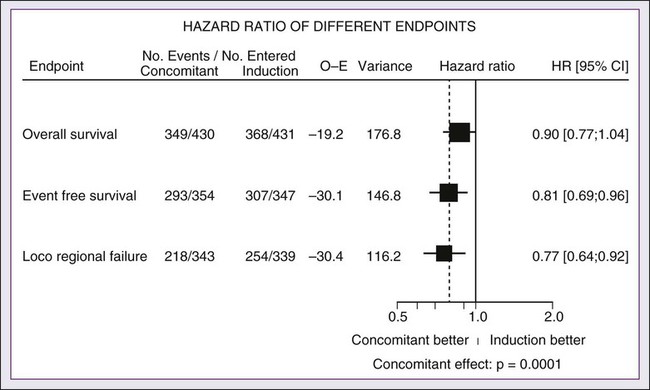
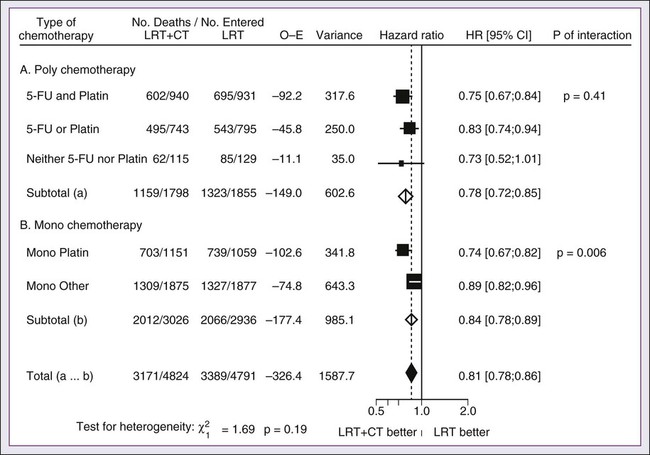
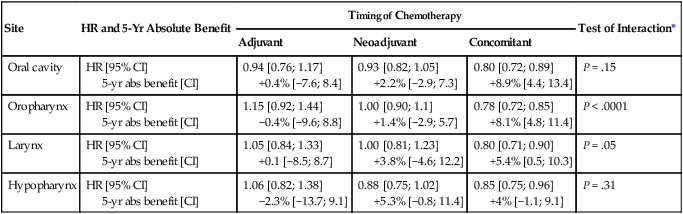
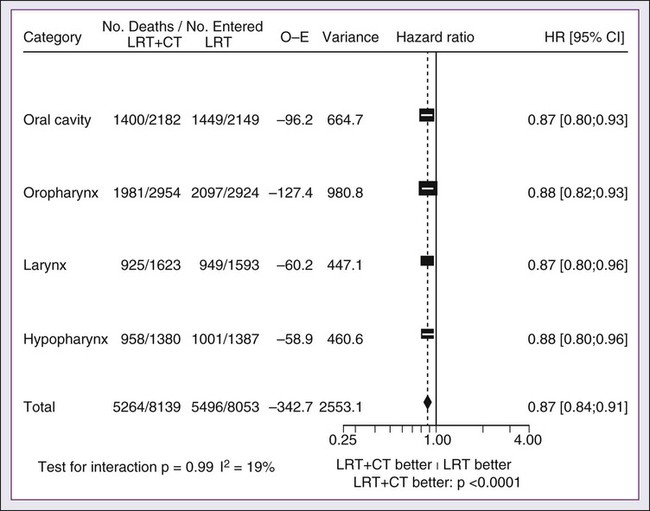
68 Paul B. Romesser, Nadeem Riaz, Alan L. Ho, Richard J. Wong and Nancy Y. Lee • In the United States it was estimated that in the year 2012, head and neck cancer would account for 3.2% of all new cancer cases and 2% of all cancer deaths. • The three major risk factors for head and neck cancer are tobacco, human papillomavirus infection, and alcohol. • Management of head and neck cancers requires a multidisciplinary approach with effective integration of multiple specialties to achieve the desired goals of cure and functional organ preservation. • Staging of head and neck cancer should be comprehensive and include a complete physical examination, fiberoptic laryngoscopy, computed tomography and/or magnetic resonance imaging of the head and neck, and a positron emission tomography scan for advanced stage disease to assess nodal involvement and distant metastases. • The treatment of head and neck cancer is dictated by the primary site. • Management of paranasal sinus malignancies is primarily surgical, with adjuvant radiation and possibly chemotherapy for advanced lesions. In unresectable and nonoperative cases, definitive radiotherapy should be offered. • Surgery is generally considered the preferred initial treatment modality for oral cavity lesions. Adjuvant radiotherapy with or without chemotherapy is indicated for patients with high-risk features on surgical pathology. Definitive radiotherapy is reserved for unresectable tumors, nonoperable tumors, and tumors in which surgical resection would result in significant functional impairment. • Most oropharyngeal cancers in the United States are now due to human papillomavirus and as such have an improved prognosis. Early-stage oropharynx cancers can effectively be treated with surgery or radiation therapy. The standard of care for locally advanced disease is chemoradiation; however, other modes of treatment are under active investigation. • In persons with larynx cancer, the goal of first-line therapy should be to preserve the function of the larynx, without sacrificing tumor control. Early-stage laryngeal cancer can effectively be treated with either surgery or radiotherapy. In early-stage disease, the anatomic location and extent of disease will dictate if surgery is feasible. The treatment of choice in most locally advanced larynx cancers is concurrent chemotherapy and radiation. Because the Veterans Affairs (VA) larynx study demonstrated poor outcomes for persons with T4a disease, total laryngectomy is also a consideration for these patients. • Surgical resection is the mainstay of management in salivary gland cancer, whether it arises from the parotid, submandibular, sublingual, or minor salivary glands. Combined therapy is recommended for high-grade malignancies of the salivary gland because postoperative irradiation has been shown to improve local-regional control in patients with positive surgical margins; high-risk features such as advanced stage, high-grade, skin/nerve invasion; and adenoid cystic carcinoma. • The management of locally recurrent head and neck cancer is technically challenging and should be performed at centers where personnel have experience in treating these patients. Surgery is typically preferred and offered for resectable lesions in the absence of unacceptable functional sequelae. The roles of adjuvant radiotherapy (or reirradiation) and chemotherapy must be determined on a case-by-case basis. • Metastatic head and neck squamous cell carcinoma carries a poor prognosis with a median survival of months. Systemic therapies are indicated for widespread disease. In the year 2012, it was estimated that head and neck cancer (HNC) would account for 3.2% of all new cancer cases and 2% of all cancer deaths in the United States.1 Because these tumors are relatively uncommon, misdiagnosis along with patient neglect contributes to an advanced stage at presentation and limited survival.2 Despite the relatively low numbers of HNCs in clinical practice, research and management of HNCs continue to receive significant emphasis because of the rich anatomic and functional complexity of this body site, which is critical to issues of self-esteem, communication, and social integration. Although squamous cell carcinomas (SCCs) constitute the majority of adult histopathological patterns in HNCs, the variation in histopathological types and the differing features of the possible anatomic subsites of involvement result in tremendous variability in the natural course of the disease for this small anatomic region.3 Tobacco, alcohol, and human papillomavirus (HPV) infection are the three major risk factors for head and neck squamous cell carcinoma (HNSCC) in developed countries. Current smokers have an approximate tenfold higher risk of HNC than do lifelong nonsmokers.4 Historically, approximately 80% to 90% of all head and neck carcinomas were attributable to tobacco consumption, particularly cigarette smoking, although currently the attribution is thought to be less given an increase in HPV-associated HNC.4 HPV-positive HNC incidence has been steadily increasing and is expected to surpass the incidence of HPV-negative HNC, which traditionally has been associated with tobacco and alcohol.5 It remains to be seen if current HPV vaccination recommendations will result in a decrease in HPV-associated HNC. Currently the American Joint Committee on Cancer (AJCC) staging system, which uses a unidimensional tumor size and local anatomic invasion (T category), nodal involvement (N category), and presence of metastatic disease (M category), is the most widely accepted and applied prognostic system relating to cancer.6,7 Generally, T1, T2, and T3 represent increasing tumor size, whereas T4 is defined by invasion of a surrounding structure (i.e., skin, nerve, vessel, and cartilage). The node (N) category is classified largely by size and location (ipsilateral vs. contralateral) of involved lymph nodes. The absence or presence of distant metastases is defined as M0 if absent or M1 if present. The T, N, and M categories are combined into overall AJCC stages that are presented in Table 68-1. Because the natural history of HNC varies somewhat according to specific anatomic location of the primary disease and because stages III and IV include a large number of different T and N stages, it is customary to refer to specific head and neck cancers by their individual T, N, and M stage and the primary site. Table 68-1 Overall Group Staging of Head and Neck Cancer: Tumor-Node-Metastasis (TNM) Classification From American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. After completion of initial treatment, patients are carefully assessed for response to treatment. For advanced-stage cancers treated with chemoradiation therapy (CRT), CT, positron emission tomography (PET), and/or MRI scans are obtained to assess radiographic disease response approximately 12 weeks after completion of treatment. Thereafter patients are seen at gradually increasing intervals for surveillance examinations. Chest radiographs may be obtained intermittently as indicated for surveillance for metastatic disease or secondary primary malignancies, particularly for patients with a history of smoking. Thyroid-stimulating hormone levels should be checked every 12 months because 20% to 25% of patients who have had neck irradiation experience hypothyroidism, necessitating thyroid hormone supplementation.8 After 5 years, patients may be examined yearly. The single-modality principle for treatment of early-stage disease attempts to minimize toxicity and emphasizes the importance of patient and tumor selection. When postoperative RT (PORT) appears likely, definitive RT may be more appropriate, particularly for small-volume disease, because it is unclear that combined modality therapy is superior to RT alone. This consideration is particularly relevant when initial definitive en bloc resection may, in combination with PORT, further complicate treatment toxicity and organ function. Not uncommonly, these issues arise with more extensive early-stage HNSCC or in cases in which the anatomic location limits achieving adequate surgical margins. For unresectable locally advanced disease, conventionally fractionated RT has been traditionally recommended. Suboptimal local-regional disease control in the absence of surgical options has forced investigation into various modalities of treatment intensification, including neoadjuvant chemotherapy, concurrent CRT, and altered fractionated RT.9,10 Although the AJCC staging system is the most widely accepted and applied prognostic system in persons with cancer, much attention has been given to its inability to identify patients with HNC who are at high risk of treatment failure.7 As such, various radiographic biomarkers have been studied to help improve our ability to prognosticate outcomes for individual patients. During the past decade, interest has expanded in the use of PET-CT, specifically given its ability to be an accurate and sensitive imaging modality for the pre- and posttreatment evaluation of patients with HNC compared with clinical examination and CT alone.11,12 Controversy has plagued studies evaluating the prognostic and predictive utility of the standardized uptake value, essentially the metabolic tumor activity. The success of metabolic-based radiographic biomarkers rests on the ability to standardize protocols allowing interinstitutional comparisons. Similarly, some volumetric indices have been explored as potential prognostic and predictive indices. Multiple studies have demonstrated that the gross tumor volume correlates with local-regional control and survival in patients with HNSCC who are undergoing surgery,13 RT,16–16 or CRT treatments in various HNC sites.17–21 Further incorporation of metabolic volume metrics in defining tumor volume, such as the metabolic tumor volume, has defined yet another prognostic radiologic biomarker.7,22,23 Unfortunately, no volumetric cut points have been prospectively validated. Typically, combined-modality strategies are used for the treatment of neck disease. In a neck with negative clinical findings, when surgical resection has been elected for the primary site management, elective neck dissection may be omitted for most subsites if preoperative evaluation determines a high risk of requiring PORT and the risk of occult nodal metastasis is sufficiently high to warrant elective management. When RT has been selected for management of the primary site, neck dissection in a neck with negative clinical findings is not indicated. In fact, pre-RT neck dissection may alter the lymphatic flow of the neck, necessitating larger volumes of the neck to be irradiated. Pre-RT neck dissection also may contribute to delays in the delivery of RT, which have been reported in a retrospective analysis to contribute to an adverse overall survival when compared with post-RT neck dissection.24,25 When conventionally fractionated RT alone has been used for locally advanced HNSCC, suboptimal regional control rates coupled with the morbidity and the limited success of subsequent salvage neck dissection have prompted the incorporation and general acceptance of a planned neck dissection. This approach, in the presence of residual adenopathy after RT, also has been selectively applied to patients with adverse risk factors such as large nodal size (typically 3 cm or greater). This risk stratification is based on RT series demonstrating an inverse relationship between nodal size and control rate.26,27 In a study of RT alone for treatment of HNSCC in 1251 patients, Dubray and colleagues27 noted 3-year neck control rates, by maximum nodal size, of 77% for 0.5 cm, 67% for 2 cm, 60% for 4 cm, 52% for 6 cm, 37% for 8 cm, and 7% for 10 cm. Multivariate analysis revealed that regional relapses independently increased with increased nodal size (P = .0001), decreased radiation dose (P = .0001), T4 primary disease (P = .0001), node fixation (P = .02), bilateral neck disease (P = .03), and geographic miss (P = .0001). Controversy continues, however, regarding the benefit of a planned neck dissection in the setting of a complete clinical response in the neck, particularly in large pretreatment lymph nodes, after completion of RT.30–30 Treatment with conventionally fractionated RT has demonstrated that the prognosis of large neck nodes with a complete response is associated with a prognosis comparable with that of smaller nodal metastases, and that the risk of relapse is low.26,31 This finding suggests that perhaps the subgroup of patients with a complete response in the neck may have more radiosensitive disease and may not require a neck dissection. A neck dissection continues to be favored for persons with advanced neck disease (especially stage N3), because the likelihood of achieving a complete response with RT alone is limited. In addition, assessment of disease response by PET/CT allows a more objective evaluation of patients who would likely benefit most from a neck dissection. Management of patients with HNSCC is further complicated by the risk for the development of second primary carcinomas and relapses within an aerodigestive tract that may have been extensively exposed to prior treatment. An irradiated aerodigestive tract not only limits reirradiation but also may preclude an effective surgical salvage, because concerns of residual microscopic disease often exist in cases that would otherwise warrant use of PORT. When a second primary or relapse occurs within a previously irradiated field, surgical resection should be the primary treatment option.32 Experiences with various repeat external beam radiotherapy (EBRT) strategies, including the integration of chemotherapy, have unfortunately demonstrated limited success, often at the risk of significant toxicities.32,33 The exception appears to be nasopharyngeal carcinomas (NPCs) that are more radiosensitive. Even then, significant late complications may arise but may need to be considered in view of the limited surgical options. A variety of different types of neck dissections may be performed (Fig. 68-1). The radical neck dissection is a procedure wherein the lymph nodes from all five levels of the neck, the sternocleidomastoid muscle, the internal jugular vein, and the spinal accessory nerve are all removed en bloc. This procedure is indicated in the setting of high-volume neck metastases involving these nonlymphatic structures. A modified radical neck dissection removes lymph nodes from all five levels of the neck but spares one or more of the nonlymphatic structures. Three types of modified radical neck dissection are type I (which spares one structure, most commonly the spinal accessory nerve), type II (which spares two structures, most commonly the spinal accessory nerve and the internal jugular vein), and type III (which spares all three structures—the spinal accessory nerve, internal jugular vein, and sternocleidomastoid muscle). A type III dissection also is termed a functional or Bocca neck dissection.34 Of these three structures, removal of the accessory nerve is generally considered to result in the most clinically significant functional deficit from shoulder dysfunction. Selective neck dissections involve the resection of fewer than all five levels of lymph nodes, usually involving three or four levels according to the site of the primary cancer. These neck dissections are commonly performed in the setting of necks classified as N0, but they may be performed in selected cases of N+ nodal disease.37–37 RT has a long and successful history in the treatment of primary head and neck malignancies. Historical experiences with EBRT have demonstrated that acute treatment-limiting RT-induced dermatitis may be limited by fractionation and the use of higher energy RT. Accordingly, current standard RT practices have evolved to utilize a fractionated RT prescription using modern linear accelerators that can produce a spectrum of beam energies. With fractionation, issues of patient immobilization and treatment set-up reproducibility become important considerations. For the head and neck, critical normal tissue structures such as the spinal cord and optic chiasm often are in close proximity to the irradiated target. For these reasons, a prerequisite for treatment simulation is that patients are immobilized with various devices such as a custom-made face mask and frame, with the setup referenced to a laser light coordinate system in the treatment rooms (Figs. 68-2 and 68-3). Immobilization addresses the issue of the precision of the treatment delivery as a strategy to optimize the therapeutic ratio. Historically, patients were treated with two-dimensional RT, which did not permit significant sparing of normal tissues and resulted in significant toxicity. In these cases, the oncologist would outline the areas to irradiate on a radiograph of the head and neck. Modern treatment with intensity-modulated RT (IMRT) involves obtaining axial CT images and delineating the tumor and normal tissue on each CT slice. IMRT allows the modulation of the RT beam to achieve exquisite dose conformality, which means that the tumor receives the full dose of RT while minimizing the dose delivered to critical normal structures. Multiple randomized trials have now demonstrated significantly reduced toxicity with IMRT compared with two-dimensional RT.40–40 Given the improved conformality, the success of IMRT is dependent on the ability to identify anatomic sites that harbor subclinical disease. Currently, the basis for this determination is derived from surgical and clinical documentation of disease extension that is often unique to each head and neck subsite. During the past decade interest has been expanding in PET/CT, specifically given its ability to be an accurate and sensitive imaging modality for the pre- and posttreatment evaluation of patients with HNC compared with clinical examination and CT alone.11,12 Currently, the radiation oncologist must incorporate data from the clinical examination, CT, PET/CT, and often MRI when designing the radiation plan. The identification of nodal groups typically at risk for harboring subclinical nodal metastases has proven more difficult. To aid in this identification, several reports have defined axial anatomic structures that may be used to delineate the various nodal groups (Table 68-2).41 The incidence of nodal metastases has been summarized by site and can aid the radiation oncologist (Table 68-3). Of note, however, when prior treatment to the neck has occurred, altered flow of lymphatics is a concern. As with the head and neck surgeon, judgment must be exercised with these precise treatment techniques. Table 68-2 Recommendation for the Radiologic Boundaries of the Neck Node Levels *Midline structure lying between the medial borders of the anterior belly of the digastric muscle. From Grégoire V, Coche E, Cosnard G, et al: Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000;56:135–50. Table 68-3 Distribution of Clinical Metastatic Neck Nodes from Head and Neck Squamous Cell Carcinomas †Ipsilateral/contralateral nodes. From Grégoire V, Coche E, Cosnard G, et al: Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000;56:135–50. Conventional or daily RT fractionation (typically, daily 1.8- to 2-Gy fractions to a total dose of 70 Gy) has permitted the delivery of high RT doses, which is currently limited by normal tissue radiation dose tolerance levels, such as in the mandible, parotid glands, brainstem, and lens. Conceptually, RT failures may result from insufficient doses of RT relative to the number of tumor clonogens or may be due to cellular mechanisms of radioresistance. The former has proved to be more amenable to therapeutic manipulation with the use of altered fractionation schedules, which may be generalized into two groups: hyperfractionation and accelerated fractionation.42 A hyperfractionation schedule, or the use of lower doses per treatment fraction, has been hypothesized to reduce the risk of late RT-induced complications associated with an increase in the total RT dose. Typically, the dose per fraction is reduced to 1.2 to 1.5 Gy and exploits a differential radiosensitivity between normal late-responding tissues and most HNSCC. To ensure that the overall treatment time is not adversely protracted, fractions often are delivered twice a day, with an interfraction period of 6 hours. European Organization for Research and Treatment of Cancer (EORTC) trial 22791 compared hyperfractionation (1.5 Gy twice daily to a total dose of 80.5 Gy) with conventional fractionation (2 Gy once daily to a total dose of 70 Gy) in patients with stage T2-3N0-1 oropharyngeal cancer (excluding the base of the tongue). This trial demonstrated a statistically significant improvement in local control of the hyperfractionation arm (38% vs. 56%, P = .01) at 5 years with no increase in late complications.43 Alternatively, an accelerated fractionation RT schedule attempts to deliver the prescribed total dose over a shorter treatment duration. This strategy was founded on observations of adverse local-regional control rates with protracted treatment durations (with conventional fractionated schedules) such that higher total doses are required to maintain the same probability of tumor control. These results have been interpreted to be consistent with a model whereby tumor clonogens surviving each daily RT fraction undergo an accelerated rate of repopulation. As a consequence, a larger tumor burden would be expected with increasing duration of treatment interruptions. It has been rationalized that by reducing the overall treatment time, the opportunity and impact of accelerated tumor repopulation would be minimized. As the severity of acute toxicities is increased, some accelerated schedules studies have attempted to modify the risk of unacceptable acute toxicities by modifying either the dose per fraction or the total dose as a strategy to achieve an acceptable therapeutic ratio. EORTC trial 22851 compared accelerated fractionation (1.6 Gy three times daily to a total dose of 72 Gy) with conventional fractionation (1.8- to 2-Gy once daily to a total dose of 70 Gy) in patients with intermediate to advanced HNCs, excluding the hypopharynx. A significantly improved 5-year local-regional control rate (P = .02) was noted, but acute and late toxicities were increased in the accelerated fractionation arm.44 To date, no single fractionation schedule has proved optimal. RTOG 90-03, a phase 3 randomized study comparing hyperfractionation, two variants of accelerated fractionation, and standard fractionation, reported that both accelerated fractionation with a concomitant boost and hyperfractionation were associated with improved local-regional control and disease-free survival compared with standard fractionation, but acute toxicity was increased.10,45 The metaanalysis of RT in carcinomas of the head and neck collaborative group pooled 15 HNSCC randomized trials for a total of 6515 patients and reported that altered fractioned schedules, hyperfractionated or accelerated RT, had a 5-year absolute survival benefit of 3.4% (hazard ratio [HR] 0.92; 95% confidence interval [CI] 0.86-0.97; P = .003) and an absolute local control benefit of 6.4% (P < .0001) when compared with standard fractionation (Fig. 68-4).46 It is important to note that the benefit was significantly higher for younger patients aged <50 years. Since RTOG 01-29 and GORTEC 99-02 both demonstrated no significant improvement in concurrent fractionated CRT versus altered fractionated CRT, altered fractionated therapy is typically reserved for patients with locally advanced disease who are not candidates for systemic therapy.47,48 For more advanced primary lesions (typically defined as stage T3 and T4 disease), increased treatment-related toxicities generally have been accepted because of their inferior local control rates. Accordingly, a combined-modality approach typically has been used. Traditionally, this approach has included surgery and RT with either preoperative or postoperative conventionally fractionated RT. A postoperative protocol generally has been favored because the RT can be delayed until more accurate delineation of the tumor extent of disease and histopathological stratification of patients requiring PORT.49 PORT has been demonstrated to provide superior outcomes in a subset of patients with HNSCC compared with preoperative RT. The indications for PORT are based on factors associated with an increased risk of local-regional recurrence after surgery, including advanced tumor stage (T3/T4), presence of a positive margin, extracapsular spread outside lymph nodes, lymphovascular invasion, perineural invasion, presence of a lymph node greater than 3 cm, and multiple positive lymph nodes. When evaluated in a randomized trial in 277 patients with supraglottic larynx and hypopharynx carcinomas (Radiation Therapy Oncology Group [RTOG] trial 73–03), PORT demonstrated superior local-regional control rates compared with preoperative RT, although the results were confounded by use of a higher dose delivered in the postoperative setting.50 Poor risk factors such as the presence of extracapsular extension or positive margins require the addition of chemotherapy to PORT. In a study of 334 patients with tumors possessing high-risk pathological features and who underwent surgery with curative intent, EORTC trial 22931, patients were randomly assigned to postoperative RT alone (66 Gy) versus PORT with concurrent cis-diamminedichloroplatinum (CDDP; cisplatin) (with a dose of 100 mg/m2 on days 1, 22, and 43 of the RT regimen).51 Postoperative concurrent CRT (POCRT) significantly improved progression-free survival (HR 0.75; 95% CI 0.56-0.99) and overall survival (HR 0.70; 95% CI 0.52-0.95) and reduced the cumulative incidence of local-regional recurrence in patients receiving POCRT compared with RT alone. Similarly, RTOG 95-01, which randomized 459 patients with high-risk pathological features to receive PORT alone (60 to 66 Gy in 30 to 33 fractions) or RT plus concurrent cisplatin (100 mg/m2 on days 1, 22, and 43), reported that POCRT yielded significantly improved rates of local-regional control (HR 0.61; 95% CI 0.41 to 0.91) and disease-free survival (HR 0.78; 95% CI 0.61 to 0.99). Not surprisingly, CRT resulted in greater grade 3 or greater acute toxicity compared with RT alone (77% vs. 34%, P < .001). Pooled analysis of these two trials suggested that PORT with cisplatin should be considered for patients with either a positive margin or extracapsular extension.52 Recent randomized trials have permitted the identification of prognostic factors adversely influencing the outcome, particularly in high-risk patients.53,54 Of greater significance, it is now clear that the time from surgery to the start of PORT and the overall treatment time of PORT are important determinants of local-regional control.55 Although brachytherapy previously had a significant role in the management of HNSCCs, its current role in the initial management of HNSCC has decreased in the modern era. With the advent of IMRT and the routine use of concurrent chemotherapy, brachytherapy has become less used in the upfront setting, although this is not true in the recurrent setting. It is important to note that studies have demonstrated good outcomes with brachytherapy implants in the definitive setting for several tumor sites, including the tonsil and soft palate,56–61 oral tongue,62–65 base of tongue,66–73 and lip.74,75 Induction chemotherapy, which has been studied for more than 3 decades, has been repeatedly associated with significant tumor shrinkage and possibly with a decrease in the risk of distant metastases.76 Unfortunately, an improvement in overall survival for induction chemotherapy over standard treatment approaches has not been definitively demonstrated. Nonrandomized phase 2 trials in the 1970s used single-agent chemotherapy based on strategies used in the recurrent and metastatic setting. These single-agent trials reported 30% to 40% response rates. Induction strategies subsequently evolved to include multiple chemotherapy regimens. The first reported trials by Wittes and colleagues77 demonstrated a 71% response rate with complete responses noted in 21% of patients using cisplatin and continuous infusion bleomycin in 21 patients. Other studies using cisplatin/bleomycin with other drugs such as hydroxyurea followed and revealed increased toxicity with no improvement in response rates or survival.78 Investigators from Wayne State University reported the first trial using neoadjuvant cisplatin with infusional 5-fluorouracil (5-FU), with an overall response rate of 88% and a complete response rate of 54%.79 Trials using carboplatin with 5-FU have also produced good rates of response.80 In 1985, the increasing interest in laryngeal preservation coupled with disappointing experiences with upfront radiotherapy for advanced disease laid the foundation for the Veterans Affairs Cooperative Studies Program to initiate a multiinstitutional randomized trial of neoadjuvant chemotherapy as an organ preservation strategy.81 Patients with previously untreated, locally advanced, but potentially resectable stage III (T2-3N1 or T3N0) or stage IV (T1-3N2-3 or T4N0-1) disease of the supraglottic or glottic larynx were randomized either to receive neoadjuvant chemotherapy or to undergo surgical resection. The chemotherapy regimen was cisplatin (100 mg/m2) with continuous-infusion 5-FU (1000 mg/m2) for 5 days every 21 days with response after two cycles used to stratify patients to either continue with an additional cycle of chemotherapy followed by RT or, for nonresponders, salvage surgery followed by PORT. In total, 332 patients were enrolled: 216 patients with T3 disease, 85 with T4 disease, and 240 patients with N0-1 disease. Laryngeal preservation was achieved in 64% of patients enrolled in the chemotherapy arm. The local failure rate was significantly higher in the chemotherapy plus irradiation arm, but the distant failure rate was significantly lower in this arm. The estimated 2-year overall survival rate was 68% in each arm. This trial demonstrated that laryngeal conservation was achievable in a significant proportion of patients with advanced laryngeal carcinoma without sacrificing overall survival. However, the incremental value of neoadjuvant chemotherapy over RT alone was not answered in this trial. These findings led to the RTOG 91-11 trial, which was conducted in patients with stage III or IV resectable disease of the larynx.82 Patients were randomized to three treatment groups: chemotherapy (cisplatin plus 5-FU) followed by RT, concurrent CRT with high-dose cisplatin as the radiosensitizer, or standard fractionated EBRT. The rate of laryngeal preservation at a median follow-up of 3.8 years was significantly higher among patients who received RT with concurrent cisplatin (84%) than in those who received induction chemotherapy followed by RT (72%, P = .005) or RT alone (67%; P < .001). Both chemotherapy regimens suppressed distant metastases and resulted in better disease-free survival than did RT alone. Despite these differences, the overall survival rates were similar in all three groups. As expected, the rate of high-grade toxic effects was greater with the chemotherapy-based regimens compared with RT alone. This study demonstrated that concurrent CRT was the preferred approach for laryngeal preservation. Efforts continue to improve on the clinical efficacy of neoadjuvant chemotherapy in hopes of achieving significant activity to yield consistent survival benefits.83 Largely based on evidence from two European studies (TAX 323 and TAX 324), the U.S. Food and Drug Administration (FDA) has approved induction chemotherapy with docetaxel (Taxotere), cisplatin (Platin), and 5-FU (TPF) in patients with inoperable and operable HNSCC.76 The TAX 323 trial was a multicenter, randomized, phase 3 trial of 358 patients with previously untreated, unresectable, locally advanced stage III and IV tumors who were randomized to cisplatin and 5-FU with or without docetaxel (TPF and PF regimens, respectively).84 Four to 7 weeks after chemotherapy, patients who did not have progressive disease underwent RT. Patients treated with TPF had a reduction in their risk of death (27%, P = .02) and improved progression-free survival (HR 0.72; P = .007). The TAX 324 trial randomly assigned 501 patients with stage III or IV HNSCC to receive either PF or TPF as induction chemotherapy followed by CRT with concurrent carboplatin (sequential therapy) in patients who did not have progressive disease. In this trial, patients who received TPF had improved overall survival (HR 0.70, P = .006) and local-regional control (P = .004).85 Benefits with TPF did come at the cost of higher rates of toxicity. Notably, neither of these trials had a control arm with standard CRT alone. The metaanalysis of chemotherapy on HNC (MACH-NC) from Pignon and colleagues86 included 31 induction chemotherapy trials for a total of 5311 patients over a median follow-up of 6.1 years. Overall, induction chemotherapy had a nonsignificant absolute survival benefit of 2.4% at 5 years (HR 0.96, 95% CI 0.90-1.02: P = .18; Fig. 68-5). No significant variation (P = .23) of the effect according to the type of chemotherapy was found. These investigators concluded that no clear evidence existed of a differential effect of induction chemotherapy on survival according to age, sex, performance, stage, or tumor site. Without randomized data demonstrating the benefit of induction chemotherapy compared with CRT alone, the use of induction chemotherapy remains an investigational approach. A significant body of literature exists, including numerous randomized trials of concurrent chemotherapy, systematically analyzed by several investigators.87–90 These independent reviews have consistently favored the concurrent integration of chemotherapy with RT. Pignon and colleagues86 from the MACH-NC have reported the largest and most recently updated of these metaanalyses. A patient-based metaanalysis of 9615 patients who underwent concomitant CRT was derived from 50 randomized trials and confirmed an absolute 5-year survival benefit of 6.5% (HR 0.81; 95% CI 0.78-0.86; Fig. 68-5). The benefit of chemotherapy was due to its effect on deaths related to HNC (HR 0.78; 95% CI 0.73-0.84), because no benefit was seen for noncancer deaths (HR 0.96; 95% CI 0.82-1.12). Similarly, an absolute benefit of 6.2% was seen in event-free survival (HR 0.79; 95% CI 0.76-0.83). This benefit was largely restricted to patients 70 years of age and younger because a statistically significant decreasing effect of chemotherapy on survival with increasing age was found (P = .003). Importantly, no significant difference was seen between monochemotherapy and polychemotherapy regimens (P = .19), and no difference was noted between different polychemotherapy subgroups (Fig. 68-6). In patients receiving monochemotherapy, the effect was significantly higher with platinum agents than with other types of monochemotherapies (Fig. 68-6). In its most recent update, the MACH-NC collaborative group published a comprehensive analysis by tumor site.91 This analysis included 4331 patients with cancer of the oral cavity, 5878 patients with oropharyngeal cancer, 3216 patients with laryngeal cancer, and 2767 patients with hypopharyngeal cancer. Overall, the addition of chemotherapy resulted in a reduction of the risk of death by 13% (Fig. 68-7), with the greater benefit for tumor sites associated with concomitant administration. The 5-year absolute overall survival benefits associated with concomitant chemotherapy were 8.9%, 8.1%, 5.4%, and 4% for oral cavity, oropharynx, larynx, and hypopharynx tumors, respectively (Table 68-4). Similarly, the 5-year absolute event-free survival benefits associated with concomitant chemotherapy are 6.9%, 8.4%, 5.4%, and 3.2% for oral cavity, oropharynx, larynx, and hypopharynx tumors, respectively. In a direct comparison of concomitant versus induction chemotherapy, MACH-NC analyzed six trials with a total of 861 patients over a median follow-up of 10.9 years.86 Patients who underwent concomitant CRT had a nonsignificant improvement in overall survival with an absolute benefit of 3.5%, significantly decreased local-regional failure, and significantly improved event-free survival compared with patients who were treated with induction chemotherapy (Fig. 68-8). Table 68-4 Hazard Ratios of Death and 5-year Absolute Benefit (Overall Survival) Associated with the Use of Chemotherapy According to Tumor Site and Chemotherapy Timing abs, Absolute; CI, 95% confidence interval; HR, hazard ratio. Cetuximab, a monoclonal IgG1 antibody against the ligand-binding domain of the epidermal growth factor receptor (EGFR), has been studied as a single agent or in combination with cisplatin in patients with HNC with promising results.92,93 Bonner and colleagues94 randomized 424 patients with stage III/IV HNC to treatment with high-dose RT alone (n = 213) or high-dose RT plus weekly cetuximab (n = 211) at an initial dose of 400 mg/m2 followed by weekly 250 mg/m2 doses for the duration of RT. RT with concurrent cetuximab resulted in a significant 32% reduction in the risk of local-regional progression, increased overall survival (HR for death 0.74, P = .03), and prolonged progression-free survival (HR for disease progression or death 0.70, P = .006). This overall survival advantage was further validated in a subsequent report on the updated 5-year analysis (HR 0.73, P = .018).95 Unfortunately, in the MACH-NC metaanalysis, concurrent CRT trials with cetuximab were limited, preventing definitive conclusions. The proximity of HNCs to the oral and esophageal mucosa results in an increased risk of treatment toxicity. Approximately 50% of patients undergoing concurrent CRT experience severe dysphagia, and the majority experience significant mucositis throughout treatment, both of which result in decreased oral intake and resultant malnutrition.96,97
Cancer of the Head and Neck
Introduction
Clinical Presentation and Patient Evaluation
Staging Investigations
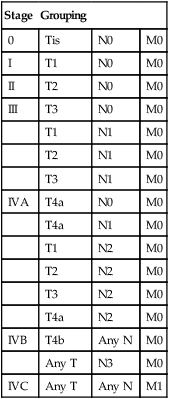
Stage
Grouping
0
Tis
N0
M0
I
T1
N0
M0
II
T2
N0
M0
III
T3
N0
M0
T1
N1
M0
T2
N1
M0
T3
N1
M0
IVA
T4a
N0
M0
T4a
N1
M0
T1
N2
M0
T2
N2
M0
T3
N2
M0
T4a
N2
M0
IVB
T4b
Any N
M0
Any T
N3
M0
IVC
Any T
Any N
M1
Follow-up Program
Treatment Overview
Defining Treatment Algorithms—Primary Site
Defining Treatment Algorithms—Management of the Neck
Second Primary Tumors
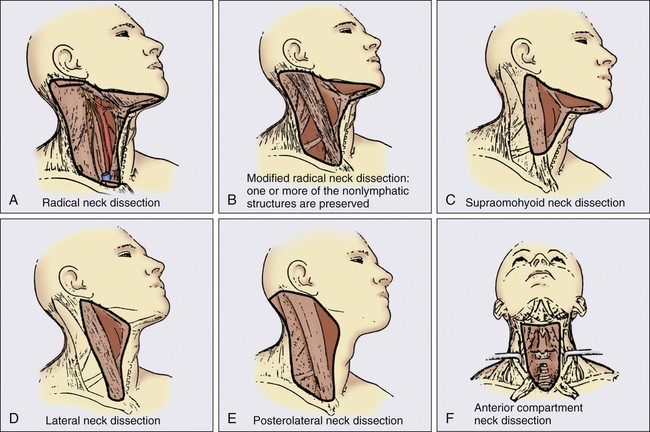
Surgery
Neck Dissection
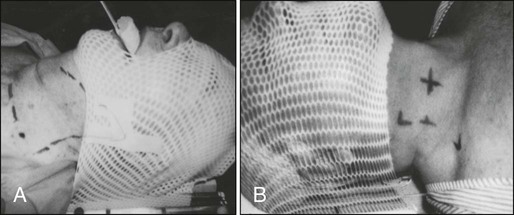
Radiotherapy
Level
Anatomic Boundary
Cranial
Caudal
Anterior
Posterior
Lateral
Medial
Ia
Geniohyoid m.
Platysma m.
Symphysis menti; platysma m.
Body of hyoid bone
Medial edge of anterior belly of digastric m.
NA*
Ib
Mylohyoid m., cranial edge of submandibular gland or caudal edge of medial pterygoid m.
Platysma m.
Symphysis menti
Body of hyoid bone; posterior edge of submandibular gland
Basilar edge of mandible; platysma m.
Lateral edge of anterior belly of digastric m.
II
Bottom edge of the body of CI
Bottom edge of the body of hyoid bone
Posterior edge of submandibular gland; posterior edge of posterior belly of digastric m.
Posterior border of sternocleidomastoid m.
Medial edge of sternocleidomastoid m.
Internal edge of internal carotid artery, paraspinal (levator scapulae) m.
III
Bottom edge of the body of hyoid bone
Bottom edge of cricoid cartilage
Posterolateral edge of sternohyoid m.
Posterior edge of sternocleidomastoid m.
Medial edge of sternocleidomastoid m.
Internal edge of carotid artery, paraspinal (scalenus) m.
IV
Bottom edge of cricoid cartilage
Cranial border of clavicle
Posterolateral edge of sternohyoid m.
Posterior edge of sternocleidomastoid m.
Medial edge of sternocleidomastoid m.
Internal edge of internal carotid artery, paraspinal (scalenus) m.
V
Skull base
Cranial border of clavicle
Posterior edge of sternocleidomastoid m.
Anterior border of trapezius m; scalenus m.
Platysma m; skin
Paraspinal (levator scapulae, splenius capitis) m.
VI
Bottom edge of the body of hyoid bone
Sternal manubrium
Skin; platysma m.
Posterolateral edge of sternohyoid m.
Medial edge of common carotid artery, skin and anterior-medial edge of sternocleidomastoid m.
NA
Retropharyngeal
Base of skull
Cranial edge of the body of hyoid bone
Levator veli palatini m.
Prevertebral m. (longus colli, longus capitis)
Medial edge of internal carotid artery
Midline
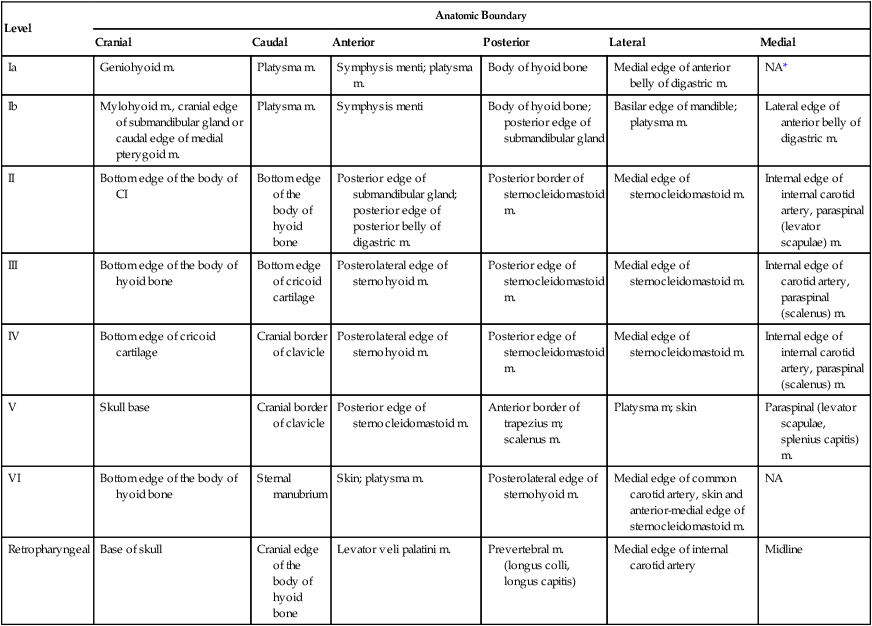
Distribution of Metastatic Lymph Nodes per Level (% of Node-Positive Patients)
Tumor Size
Patients with N+ (%)
I
II
III
IV
V
Other*
Oral cavity (N = 787)
36
42/3.5†
79/8
18/3
5/1
1/0
1.4/0.3
Oropharynx (N = 1479)
64
13/2
81/24
23/5
9/2.5
13/3
2/1
Hypopharynx (N = 847)
70
2/0
80/13
51/4
20/3
24/2
3/1
Supraglottic larynx (N = 428)
55
2/0
71/21
48/10
18/7
15/4
2/0
Nasopharynx (N = 440)
80
9/5
71/56
36/32
22/15
32/26
15/10
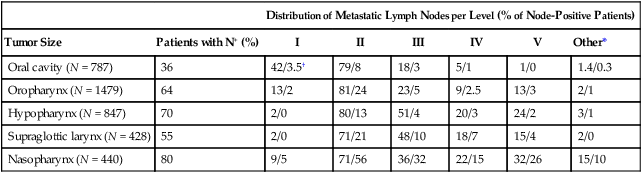
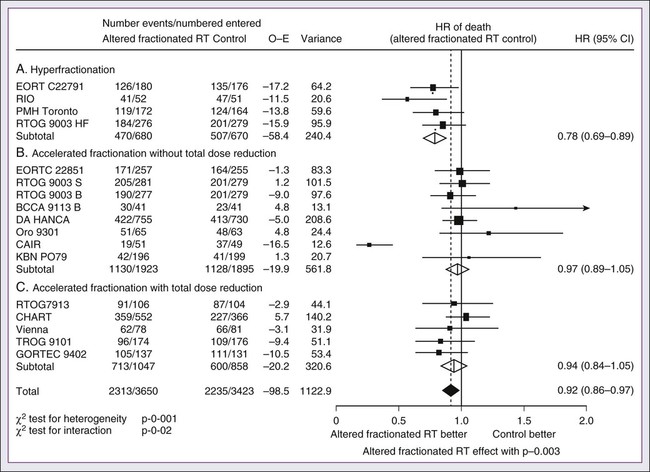
Postoperative Radiotherapy
Brachytherapy
Neoadjuvant and Induction Chemotherapy
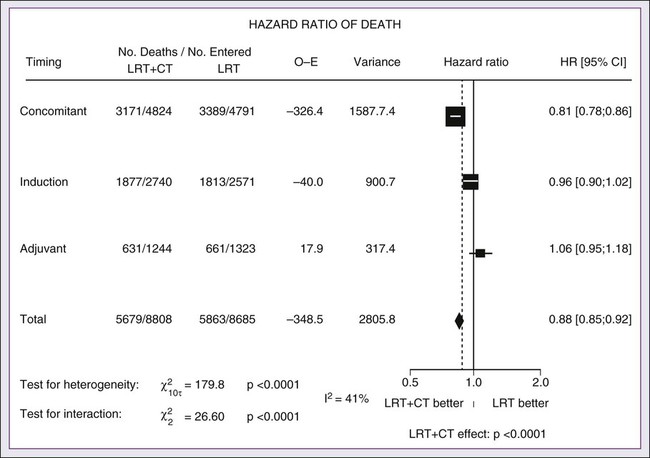
Concurrent and Concomitant Chemoradiotherapy
Site
HR and 5-Yr Absolute Benefit
Timing of Chemotherapy
Test of Interaction*
Adjuvant
Neoadjuvant
Concomitant
Oral cavity
HR [95% CI]
5-yr abs benefit [CI]
0.94 [0.76; 1.17]
+0.4% [−7.6; 8.4]
0.93 [0.82; 1.05]
+2.2% [−2.9; 7.3]
0.80 [0.72; 0.89]
+8.9% [4.4; 13.4]
P = .15
Oropharynx
HR [95% CI]
5-yr abs benefit [CI]
1.15 [0.92; 1.44]
−0.4% [−9.6; 8.8]
1.00 [0.90; 1.1]
+1.4% [−2.9; 5.7]
0.78 [0.72; 0.85]
+8.1% [4.8; 11.4]
P < .0001
Larynx
HR [95% CI]
5-yr abs benefit [CI]
1.05 [0.84; 1.33]
+0.1 [−8.5; 8.7]
1.00 [0.81; 1.23]
+3.8% [−4.6; 12.2]
0.80 [0.71; 0.90]
+5.4% [0.5; 10.3]
P = .05
Hypopharynx
HR [95% CI]
5-yr abs benefit [CI]
1.06 [0.82; 1.38]
−2.3% [−13.7; 9.1]
0.88 [0.75; 1.02]
+5.3% [−0.8; 11.4]
0.85 [0.75; 0.96]
+4% [−1.1; 9.1]
P = .31
Nutritional Considerations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine