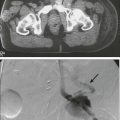
74 Lawrence Kleinberg, Ronan Kelly, Stephen Yang, Jean S. Wang and Arlene A. Forastiere • Esophageal cancer is subdivided into the following four groups: epithelial tumors, metastatic tumors, lymphomas, and sarcomas. • Cancers of epithelial origin, predominantly squamous cell and adenocarcinomas, are the most common, and other histologic types are rare. • The appropriate categorization of gastroesophageal junction tumors has been controversial, and patients have been included in clinical trials directed both at esophageal and gastric cancers. • Within the United States, the incidence of esophageal cancer in persons younger than 80 years is 3.2 per 100,000 persons. • Historically and internationally, squamous cell tumors are the most common histologic type; however, a dramatic increase in the incidence of adenocarcinoma has been documented in the United States, United Kingdom, and Western Europe. • The data support the hypothesis that epithelial tumors arise as a result of chronic irritation from a wide variety of sources, including gastric contents in chronic reflux and known carcinogens. • A strong association of Barrett esophagus and adenocarcinoma is seen, but a benefit to screening endoscopy for those at risk for or with known Barrett esophagus is unknown as the overall risk of cancer-related mortality is low. Studies with longer-term follow-up are needed to clarify this issue. Other identified risk factors are gastroesophageal reflux disease (GERD), obesity, and smoking. • Squamous cell carcinoma is associated with smoking as well as alcohol use, and the declining incidence has paralleled the decline in smoking. • Point mutations, increased copy number, and promotor region hypermethylation all appear important in the progression to malignancy. • Symptoms and demographics will strongly suggest the diagnosis. • Endoscopy is the best screening examination but esophagram may also be used. • Diagnosis is made by endoscopy with cytology and biopsy of tumor. • Transesophageal ultrasound should be used to assess T and N stage to guide optimal definitive therapy. • Computed tomography (CT) of chest and abdomen is useful in screening for metastatic disease. • Positron emission tomographic (PET) scan is useful to detect additional cases of metastatic disease before costly and toxic definitive therapy. It may be superior to endoscopic ultrasound (EUS) in detecting intraabdominal lymph nodes, but not periesophageal nodes adjacent to the primary tumor. • Additional studies include laparoscopy, thoracoscopy, bone scan, and CT of the brain when indicated by clinical circumstances. • The new AJCC/UICC 7 staging system contains important changes: adenocarcinoma and squamous cell carcinoma are separate; grade of histology is incorporated; the gastroesophageal junction is defined; nodal staging is based on number of involved nodes, similar to gastric cancer; Tis includes high graded dysplasia; and T4 is subcategorized by features suggestive of resectability. • Staging is based on pathological findings at the time of resection, but therapy is often guided by staging estimated by clinical testing. • Treatment of premalignant dysplasia is guided by grade of histology. Low-grade dysplasia should be closely followed by endoscopy. High-grade dysplasia is treated with endoscopic therapy or esophagectomy, although close follow-up may be appropriate for selected patients. • Selection of appropriate treatment for carcinoma depends on tumor stage and patient performance status. • Surgery is an accepted single-modality therapy for patients with early localized disease (T1-2N0M0) or for patients who may not tolerate combined-modality therapy. The selection of surgical approach depends upon location and experience, but no approach has been demonstrated to lead to superior cure rates. • Combined chemoradiation leads to prolonged median survival and long-term survival compared with radiation alone used as a definitive nonoperative approach, at the price of increased toxicity. This represents a potentially curative alternative to surgery for squamous cell cancers and is appropriate for most unresectable T4N and M0 lesions of either histology. Because most patients treated on prospective chemoradiation trials had squamous cell carcinomas, the benefits of nonoperative management for adenocarcinoma are not known. • Randomized trials have not confirmed a survival benefit with surgery added to potentially curative chemoradiation in squamous cell carcinoma, but there was a significant local control benefit. This question has not been well studied for adenocarcinoma, for which definitive chemoradiation is of uncertain curative potential. • Accumulating evidence convincingly demonstrates that combination therapy with preoperative chemoradiation followed by surgery survival compared with surgery alone for both locally advanced (clinically staged T2-T4 or node positive) adenocarcinoma and squamous cell carcinoma. There is less certainty about the value of preoperative chemotherapy alone. • Postoperative adjuvant chemotherapy or chemoradiation is less well studied in locally advanced esophageal cancer, but trials in gastric cancer including gastroesophageal junction adenocarcinoma have demonstrated a benefit. • Combined-modality chemotherapy regimens frequently include 5-fluorouracil (5-FU) or paclitaxel and platinum agents; other commonly used regimens include docetaxel and irinotecan. • Endoscopic palliative therapy includes laser or electrical fulguration, mucosal resection, photodynamic therapy (PDT), or stenting. Excepting very superficial lesions, these therapies are not alternatives to surgery as they do not address deeper disease or lymphatic spread. • Radiation therapy, with or without chemotherapy, may be used to palliate local symptoms. • Chemotherapy may be used for metastatic disease, but response rates and duration of response are modest for most patients. Clinical trials are recommended. Esophageal cancer (Table 74-1) is classified based on histologic appearance and cell of origin, as follows: (1) epithelial tumors, (2) metastatic tumors, (3) lymphomas, and (4) sarcomas. Cancers of epithelial cell origin, predominantly squamous cell carcinoma and adenocarcinoma, are the most common. Although squamous cell carcinoma and adenocarcinoma were previously treated as similar entities and grouped together, there is increasing recognition that they should be studied as separate entities whose optimal therapy may diverge in the era of multimodality treatments. Table 74-1 Classification of Esophageal Cancer Epithelial Squamous cell Ordinary squamous cell Verrucous squamous cell Spindle cell (carcinosarcoma) Adenocarcinoma Ordinary Adenoacanthoma Mucoepidermoid Adenoid cystic Small cell Melanoma Choriocarcinoma Metastatic disease Lymphoma Sarcoma Squamous cell cancer usually occurs in the middle third of the esophagus. In a collective review of more than 28,000 cases of squamous cell cancers, Postlethwaite1 estimated the ratio of upper, middle, and lower cancers to be 15 : 50 : 35, respectively. Adenocarcinoma, on the other hand, is most common in the lower third of the esophagus. In a collective review of 4783 cases of esophageal adenocarcinoma, Ming2 noted an upper esophageal location in 4%, middle in 18%, and lower in 67%. Of the rarer primary histologic types, 95% of small cell cancers occur in the middle and lower thirds; both malignant melanoma and choriocarcinoma tend to occur in the lower third. Esophageal sarcomas may occur anywhere along the esophagus as is the case for esophageal lymphomas or metastases from other primary cancers. Tumors of the esophagus other than squamous cell and adenocarcinoma are quite rare. This chapter, therefore, focuses on esophageal squamous cell and adenocarcinoma. Epidemiologic data show that the incidence of esophageal cancer varies considerably from one country to another and often within a single country. This geographic diversity underscores the multifactorial etiologies of esophageal cancer worldwide of which more than 90% are squamous cell cancers. In the United States and other Western industrialized countries,3–9 however, over the past 3 decades there has been a slight decline in squamous cell esophageal cancer likely the result of decreased smoking. However, there has been a dramatic rise in adenocarcinoma of the distal esophagus and GEJ, especially in some Western nations likely related to diet and increased obesity. This histology has increased in incidence approximately sixfold and since the mid-1990s has been the predominant esophageal cancer in Caucasians.7 The absolute incidence in the United States has increased from 3.8 per million in 1973 to 1975 to 23.3 per million in 2001 based on the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database. This rate of increase exceeds that of all other cancers, including lung, breast, prostate, and melanoma. Adenocarcinoma is much less common in African Americans but has increased from 0.4/100,000 to 0.9/100,000, and 0 to 0.2/100,000 in females. In recent years, it appears that although the rate of increase may be slowing in the United States, the incidence of esophageal adenocarcinoma has continued to increase at least through the year 2009 to 25.8 per million,10 with additional evidence that the incidence of GEJ adenocarcinoma in particular may be stabilizing.11 Obesity and gastroesophageal reflux disease (GERD) appear to contribute to this rise in adenocarcinoma incidence. In contrast, the incidence of squamous cell carcinoma decreased in all of these groups during this period, perhaps because of a decline in the prevalence of smoking and increased consumption of fresh fruits and vegetables.12 Two major risk factors for esophageal squamous cell carcinoma are alcohol use and tobacco smoking, according to epidemiologic studies from various countries worldwide. This relationship is dose-dependent and there is a multiplicative interaction of alcohol intake and tobacco use. In a prospective cohort study from the Netherlands involving more than 120,000 people with 16 years of follow-up, the strongest risk for squamous cell carcinoma was alcohol consumption with a 4.6-fold increased risk, whereas combined exposure with smoking increased the risk more than 8-fold. The risk of squamous cell carcinoma decreased with smoking cessation and alcohol abstinence but only after 1 to 2 decades.13 In adenocarcinoma, smoking but not alcohol is associated with increased risk. A prospective study of tobacco, alcohol, and risk of esophageal cancer in the United States found an increased risk of squamous cell carcinoma among current smokers compared with nonsmokers (hazard ratio: 9.27, 95% confidence interval [CI]: 4.04, 21.29) and also an increased risk for adenocarcinoma (hazard ratio: 3.70, 95% CI: 2.20, 6.22). For people who consumed more than three alcoholic drinks a day compared with one drink, there was increased risk of esophageal squamous cell carcinoma but not adenocarcinoma.14 A pooled analysis from an international consortium composed of 10 population-based case-control studies and 2 cohort studies found strong associations between smoking and adenocarcinoma (odds ratio [OR]: 2.08, 95% CI: 1.83, 2.37).15 However, alcohol was not found to be a risk factor for adenocarcinoma.16 Nutritional and dietary factors have been found to contribute to the risk of both squamous cell carcinoma and adenocarcinoma. Pickled vegetables, processed meat, and other foods containing nitrates have been linked to an increased risk of squamous cell carcinoma. Drinking very hot liquids or eating hot foods such as stewed meat frequently is postulated to cause thermal injury to the esophagus and has been found to be a risk factor for squamous cell carcinoma. Low consumption of fruits and vegetables are risk factors for both squamous cell carcinoma and adenocarcinoma.17 Obesity, defined as a body mass index of 30 or greater, is a strong risk factor for esophageal adenocarcinoma, as demonstrated in a meta-analysis of epidemiologic studies (OR: 2.78, 95% CI: 1.85, 4.16).18 This has clear implications for the increasing obesity rates in the United States and could in part explain the rising incidence of esophageal adenocarcinoma. The exact mechanism for this association of obesity is not understood but might reflect an increased propensity for gastroesophageal reflux. Obesity does not appear to be a risk factor for squamous cell carcinoma. Gastroesophageal reflux disease is a well-established risk factor for esophageal adenocarcinoma. A population-based case-control study in Sweden demonstrated an OR of 7.7 (95% CI: 5.3, 11.4) for development of esophageal cancer in patients with chronic reflux disease. With longstanding, severe symptoms, the OR for esophageal adenocarcinoma was 43.5 (95% CI: 18.3, 103.5).19 There was no association seen between reflux and squamous cell carcinoma. Interestingly, the increased risk of esophageal adenocarcinoma existed whether or not Barrett esophagus could be identified, leading the authors to speculate that the area of Barrett esophagus in these patients was overgrown by tumor. This theory has been supported by postchemotherapy studies showing a high prevalence of Barrett esophagus in adenocarcinoma patients. The role of screening for Barrett esophagus is controversial. A nationwide population-based study in Denmark found that patients with known Barrett esophagus comprised only 7.6% of all diagnosed esophageal adenocarcinoma patients.20 Similarly, an analysis of U.S. administrative data found that only 8% of adenocarcinoma patients had been recognized to have Barrett esophagus prior to their cancer diagnosis.21 Therefore, the value of screening endoscopy in the setting of GERD has been questioned by those who point out that Barrett esophagus is uncommon, progression to malignancy is infrequent, and the effect of screening on overall population mortality from esophageal adenocarcinoma appears quite low. A large nationwide case-control study in Sweden found that among patients with adenocarcinoma, 62% had histologic evidence of Barrett esophagus but 40% did not have a history of gastroesophageal reflux.19 Thus screening endoscopy of individuals with symptomatic gastroesophageal reflux will still miss the substantial proportion of individuals with esophageal adenocarcinoma who do not report reflux symptoms. The American Gastroenterological Association and American College of Gastroenterology guidelines state that there is currently insufficient evidence to recommend routine screening for Barrett esophagus in patients with gastroesophageal reflux symptoms and that the decision to screen a patient should be individualized.22,23 The prevalence of Barrett esophagus is estimated to be 1% to 2% of the general population.24 The length of time for progression from Barrett esophagus to dysplasia to adenocarcinoma is unknown. Many advocate lifelong endoscopic surveillance for patients with Barrett mucosa with the goal of treating dysplastic changes and thereby reducing the risk of developing adenocarcinoma. Indeed, tumors that are discovered during surveillance appear to be of an earlier stage and therefore to have a higher chance of cure.25 Still, it is not certain whether surveillance reduces mortality; the overall risk of death from esophageal cancer is relatively low even in this high-risk population. Furthermore, surveillance of Barrett esophagus patients with upper endoscopy is fraught with the problem of sampling error when biopsies are performed during endoscopy and to a high degree of interobserver variability in dysplasia grading. Several centers have reported results of endoscopic surveillance programs that suggest that progression of Barrett esophagus to adenocarcinoma might be less common than was originally thought, at least in the short term. A nationwide population-based cohort study in Denmark followed up with more than 11,000 patients with Barrett esophagus for a median of 5.2 years and found that the annual risk of adenocarcinoma was 0.12% (95% CI: 0.09, 0.15).20 Meanwhile, a meta-analysis of 57 studies involving more than 11,000 patients with nondysplastic Barrett esophagus found a pooled annual incidence rate of adenocarcinoma of 0.33%.26 Patients with low-grade dysplasia had an incidence rate of adenocarcinoma of 0.5% per year, whereas those with high-grade dysplasia have an incidence rate of adenocarcinoma of 3% to 5% per year. Molecular genetic data support the histologic observation that there is a progression from normal epithelium to Barrett esophagus to dysplasia to adenocarcinoma.27–32 Although a clearly defined sequence of genetic alterations leading to adenocarcinoma has not been defined, an accumulation of abnormalities27,33–35 has been identified in a wide range of genes that regulate proliferation, apoptosis, invasion, metastasis, angiogenesis, growth, and cell cycle regulation. Tumor suppressor genes have been implicated as early events, as loss of cell cycle checkpoints may be permissive for genetic instability, allowing later transformation in the metaplasia–dysplasia–adenocarcinoma sequence. In the last decade,36 epigenetic modifications have emerged as heritable and fundamental features of most malignancies, including esophageal adenocarcinoma.37–42 The best-studied epigenetic modification of the DNA is promoter region hypermethylation, an epigenetic modification that is associated with gene inactivation. A meaningful understanding of the molecular events that result in progression to adenocarcinoma will likely require a greater understanding of this phenomenon as it occurs at least as frequently as point mutations. In oncogenesis, hypermethylation is often associated with inactivation of tumor suppressor genes, of genes that suppress metastasis and angiogenesis, as well as of genes that repair DNA. Methylation of DNA occurs mostly at CpG sites in the genome and is catalyzed by a family of three active DNA methyl-transferases that transfer a methyl group from S-adenosyl-methionine to cytosine to form 5-methylcytosine. Because this reaction can be blocked effectively by a drug, 5-azacytidine, which acts as an irreversible inhibitor of the DNA methyltransferases, the therapeutic potential inherent in reversing DNA hypermethylation is significant.43 One of the earliest events that is thought to occur in the molecular progression of Barrett esophagus to adenocarcinoma is inactivation of one of the alleles of the tumor suppressor gene p16 via DNA hypermethylation, loss of heterozygosity (LOH), or mutations. This event is thought to be triggered by chronic inflammation secondary to acid and bile reflux and has been found to occur at the stage of Barrett esophagus with no dysplasia. The p16 gene is located on the short arm of chromosome 9 and is a cyclin-dependent kinase inhibitor which regulates the cell cycle.29,30,44,45 When p16 is inactivated, this promotes phosphorylation of the retinoblastoma protein, leading to proliferation. This clone of cells may expand and subsequently there may be loss of the second p16 allele as a result of LOH and the formation of several p16 null clones. When Barrett cells begin to acquire the hallmarks of cancer, there is clonal expansion of cells that have a selective growth advantage because of the genetic or epigenetic changes they have acquired. Studies have also documented the importance of p53 inactivation,46–49 the loss of one allele of the tumor suppressor gene, p53, via mutation. Later, loss of the second allele of p53 via LOH may occur, resulting in the inactivation of p53. The gene p53 is located on the short arm of chromosome 17 and is involved in regulating cell cycle control. When cells sustain DNA damage and cannot be repaired, p53 is responsible for inducing apoptosis and preventing the replication of genetic instability. Abnormalities in p53 usually occur when one allele has been deleted (usually via mutation) and the other allele is functionally inactivated, often because of LOH in a two-hit mechanism. Therefore, inactivation of p53 removes the ability to repair DNA damage and leads to the replication of genetic instability. Missense mutations of the p53 gene cause the protein to have a much longer half-life than normal, resulting in accumulation in the nucleus, where its overexpression can then be detected by immunohistochemical staining. However, p53 staining has been found to be inaccurate in some cases, leading to high false-positive and false-negative rates. Meanwhile, detection of p53 LOH appears to be a more accurate marker of p53 gene abnormalities and has been found to be a predictor of progression to cancer. The loss of p53 is thought to be involved in the progression from Barrett esophagus with no dysplasia to low-grade dysplasia. The inactivation of p53 leads to loss of cell cycle regulation, which may promote genomic instability and aneuploidy, leading to additional changes required for progression to high-grade dysplasia and malignancy. Genomic instability is detected via DNA content abnormalities such as aneuploidy, which refers to gains or losses in parts of chromosomes. Aneuploidy in fact has been one of the most studied markers for neoplastic progression in Barrett esophagus.50 Several prospective studies using flow cytometry within a large cohort of Barrett esophagus patients have demonstrated the presence of aneuploidy during the progression from Barrett esophagus to adenocarcinoma. This promotes the formation of multiple clones, especially as the degree of genetic instability increases, and this clonal diversity ultimately leads to the development of cancer.22 Several recent studies have found that a combination panel of biomarkers is a better predictor of progression to adenocarcinoma than individual biomarkers alone. Recently, a prospective study of Barrett esophagus patients found that a combination of DNA content abnormalities (tetraploidy and aneuploidy), p16 LOH, and p53 LOH provided the best prediction of risk for adenocarcinoma (RR: 38.7, 95% CI: 10.8, 138.5). Patients with all of these findings had at least a 79% adenocarcinoma risk over 10 years, whereas patients with none of these findings had only a 12% risk of adenocarcinoma over 10 years.51 In another prospective study of Barrett esophagus patients, the combination of genetic instability and clonal expansion predicted progression to adenocarcinoma. The investigators defined the size of the clone (x) as the length of the Barrett abnormality multiplied by the portion of the cells in the biopsy flow cytometry specimens that carry the lesion. Taking into account the sizes of clones assessed in this way, relative risks for progression to adenocarcinoma, in comparison with those without the clonal abnormalities, were determined. For p53 LOH, the relative risk (RR) was 1.27x for an x cm clone (95% CI: 1.07, 1.50) and for aneuploidy/tetraploidy the RR was 1.31x (95% CI: 1.07, 1.60). A 5-cm clone containing either p53 LOH or aneuploidy/tetraploidy was associated with an RR of 4.16 (95% CI: 2.01, 8.95) for progression to adenocarcinoma.33 There appears to be a diversity of molecular abnormalities in esophageal cancer caused by actual genetic mutations, epigenetic inactivation, and altered cell regulation. The method of identifying and codifying alterations in these genes into a clinically useful paradigm has not yet proved superior to standard histology for predicting outcome, but more complex analyses using sophisticated molecular techniques such as genomic arrays could be helpful in the future. In the meantime, new molecular abnormalities continue to be identified.52 Endoscopic approaches may be curative for very early lesions with no more than superficial submucosal invasion, which are also curable with surgical resection and often radiation. Other early lesions, stage I-IIa, may be appropriately treated with esophagectomy as a single modality for suitable operative candidates. There has been substantial controversy about the optimal management of more advanced but still curable (localized) esophageal cancer where the treatment options include surgery alone, chemotherapy followed by surgery or adjuvant chemotherapy after resection, chemoradiation followed by surgery, and definitive chemoradiation. Radiation alone as definitive treatment aimed at cure is inferior to combined chemoradiation for locally advanced disease and should only be considered when the other options are not feasible. Similarly, radiotherapy used as a single adjuvant or neoadjuvant therapy has not been shown to improve outcome. These options are summarized in Table 74-2. Table 74-2 Options in the Therapy of Esophageal Carcinoma AC, Adenocarcinoma of the distal esophagus; SCC, squamous cell carcinoma. The long-term survival outcome of patients with more locally advanced disease enrolled in selected prospective trials reporting 5-year survival outcomes is summarized in Table 74-3 according to treatment approach. Although patient selection factors may have differed among the trials, there has tended to be better outcome with use of neoadjuvant therapy, either chemoradiotherapy or chemotherapy, compared with the outcome of surgery alone. The benefit of this approach for locally advanced esophageal cancer has been confirmed in randomized trials and is increasingly accepted as the optimal approach. Although never definitively compared in a completed randomized trial, these long-term results suggest a greater benefit to combined neoadjuvant chemoradiation compared with chemotherapy alone, an observation supported by data summarized below. Although radiation as a single therapy is very rarely curative and is generally only useful for palliation, definitive chemoradiation has been demonstrated to be a potentially curative alternative to surgery for locally advanced squamous cell carcinoma, and is appropriate for patients with adenocarcinoma as well who have unresectable primary tumors or inoperable disease because of medical co-morbidities. Adjuvant therapy has been less extensively studied but may be appropriate for patients with locally advanced disease who did not receive preoperative therapy. There are no data to support the concept that chemoradiation is useful to convert an unresectable lesion into a resectable lesion. Table 74-3 Published Prospective Series With 5-Year Follow-up *Patient either had adenocarcinoma (adeno) or squamous cell carcinoma. R0 is complete resection with negative margins. Given the lack of randomized comparative data to demonstrate superiority of any one of the combined-modality approaches for resectable disease, clinical decision making for optimal management of locally advanced disease remains complex and controversial. Previously available data from phase II trials, underpowered phase III trials, and metaanalyses described below indicate improved local control and suggest a survival benefit on the order of 10%, which lead to acceptance of the use of neoadjuvant chemoradiation. Recently the results have become available for the well-powered Chemoradiotherapy for Esophageal Cancer Followed by Surgery Study (CROSS)53 trial, described below, which has definitively demonstrated a survival benefit to neoadjuvant paclitaxel, carboplatin, and radiotherapy in a population largely consisting of patients with adenocarcinoma. Commonly used preoperative chemoradiation regimens are associated with substantial toxicity, and therefore, trimodality therapy should be used cautiously in patients with poor performance status or co-morbid conditions that increase the risk of life-threatening toxicity. Such patients might be better treated with surgery alone, or with combined chemoradiation (no surgery) for squamous cell carcinoma, for which this has been well demonstrated to be a potentially curative alternative to resection. The updated AJCC/International Union Against Cancer (UICC) 7th edition staging system was implemented in 2010.56–56 The 7th edition staging meaningfully differs from the 6th edition AJCC/UICC system. These staging systems and their differences are summarized in Tables 74-4 through 74-6. An important limitation similar to both systems is that they are based on surgical pathology, whereas many patients are treated with neoadjuvant therapies based on clinical and radiographic estimation of the stages. Not only is clinical staging subject to more uncertainty and error, but the use of preoperative therapy may change the prognostic significance of pathological staging criteria in those who are downstaged as a result of response to therapy and in those who progress during treatment. The trial results reported in this chapter are based on the AJCC/UICC 6th or earlier editions of the staging system in effect until recently. Survival by stage for adenocarcinoma and squamous cell carcinoma patients is summarized in Figure 74-1, for patients included in a worldwide database of 4627 surgically treated patients.57 Table 74-4 TNM Staging for Esophagus: Comparison of AJCC 6th and 7th editions
Cancer of the Esophagus
Classification and Location
Incidence
Pathogenesis
Clinical Risk Factors
Adenocarcinoma: Role of GERD and Barrett Esophagus
Molecular Progression to Adenocarcinoma
Overview: the Choice of Therapy
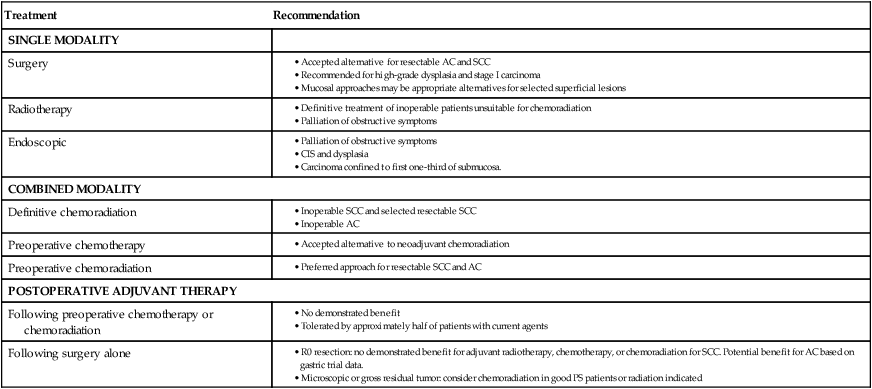
Treatment
Recommendation
SINGLE MODALITY
Surgery
Radiotherapy
Endoscopic
COMBINED MODALITY
Definitive chemoradiation
Preoperative chemotherapy
Preoperative chemoradiation
POSTOPERATIVE ADJUVANT THERAPY
Following preoperative chemotherapy or chemoradiation
Following surgery alone
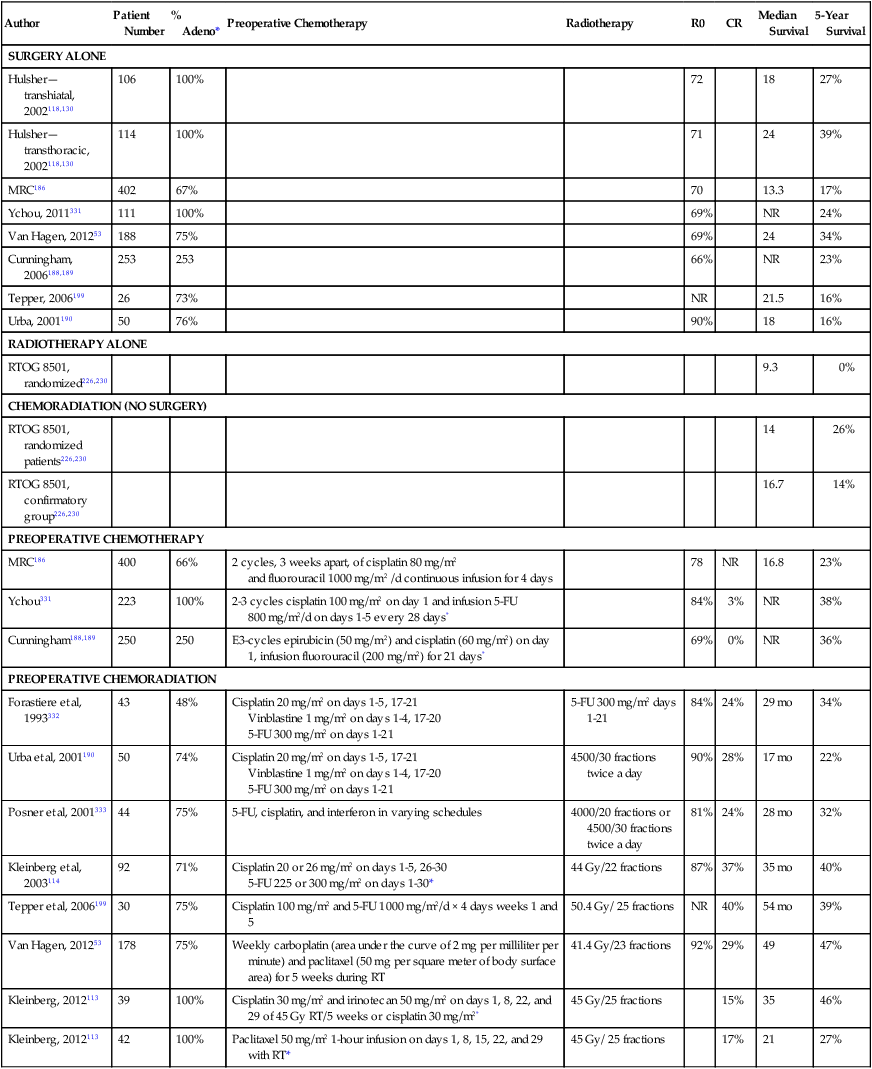
Author
Patient Number
% Adeno*
Preoperative Chemotherapy
Radiotherapy
R0
CR
Median Survival
5-Year Survival
SURGERY ALONE
Hulsher—transhiatal, 2002118,130
106
100%
72
18
27%
Hulsher—transthoracic, 2002118,130
114
100%
71
24
39%
MRC186
402
67%
70
13.3
17%
Ychou, 2011331
111
100%
69%
NR
24%
Van Hagen, 201253
188
75%
69%
24
34%
Cunningham, 2006188,189
253
253
66%
NR
23%
Tepper, 2006199
26
73%
NR
21.5
16%
Urba, 2001190
50
76%
90%
18
16%
RADIOTHERAPY ALONE
RTOG 8501, randomized226,230
9.3
0%
CHEMORADIATION (NO SURGERY)
RTOG 8501, randomized patients226,230
14
26%
RTOG 8501, confirmatory group226,230
16.7
14%
PREOPERATIVE CHEMOTHERAPY
MRC186
400
66%
2 cycles, 3 weeks apart, of cisplatin 80 mg/m2
and fluorouracil 1000 mg/m2 /d continuous infusion for 4 days
78
NR
16.8
23%
Ychou331
223
100%
2-3 cycles cisplatin 100 mg/m2 on day 1 and infusion 5-FU 800 mg/m2/d on days 1-5 every 28 days*
84%
3%
NR
38%
Cunningham188,189
250
250
E3-cycles epirubicin (50 mg/m2) and cisplatin (60 mg/m2) on day 1, infusion fluorouracil (200 mg/m2) for 21 days*
69%
0%
NR
36%
PREOPERATIVE CHEMORADIATION
Forastiere et al, 1993332
43
48%
Cisplatin 20 mg/m2 on days 1-5, 17-21
Vinblastine 1 mg/m2 on days 1-4, 17-20
5-FU 300 mg/m2 on days 1-21
5-FU 300 mg/m2 days 1-21
84%
24%
29 mo
34%
Urba et al, 2001190
50
74%
Cisplatin 20 mg/m2 on days 1-5, 17-21
Vinblastine 1 mg/m2 on days 1-4, 17-20
5-FU 300 mg/m2 on days 1-21
4500/30 fractions twice a day
90%
28%
17 mo
22%
Posner et al, 2001333
44
75%
5-FU, cisplatin, and interferon in varying schedules
4000/20 fractions or 4500/30 fractions twice a day
81%
24%
28 mo
32%
Kleinberg et al, 2003114
92
71%
Cisplatin 20 or 26 mg/m2 on days 1-5, 26-30
5-FU 225 or 300 mg/m2 on days 1-30*
44 Gy/22 fractions
87%
37%
35 mo
40%
Tepper et al, 2006199
30
75%
Cisplatin 100 mg/m2 and 5-FU 1000 mg/m2/d × 4 days weeks 1 and 5
50.4 Gy/ 25 fractions
NR
40%
54 mo
39%
Van Hagen, 201253
178
75%
Weekly carboplatin (area under the curve of 2 mg per milliliter per minute) and paclitaxel (50 mg per square meter of body surface area) for 5 weeks during RT
41.4 Gy/23 fractions
92%
29%
49
47%
Kleinberg, 2012113
39
100%
Cisplatin 30 mg/m2 and irinotecan 50 mg/m2 on days 1, 8, 22, and 29 of 45 Gy RT/5 weeks or cisplatin 30 mg/m2*
45 Gy/25 fractions
15%
35
46%
Kleinberg, 2012113
42
100%
Paclitaxel 50 mg/m2 1-hour infusion on days 1, 8, 15, 22, and 29 with RT*
45 Gy/ 25 fractions
17%
21
27%
Staging and Diagnosis: American Joint Committee on Cancer/International Union Against Cancer 7, a Substantially Revised Staging System
AJCC/UICC 6th Edition
AJCC/UICC 7th Edition
PRIMARY TUMOR (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access