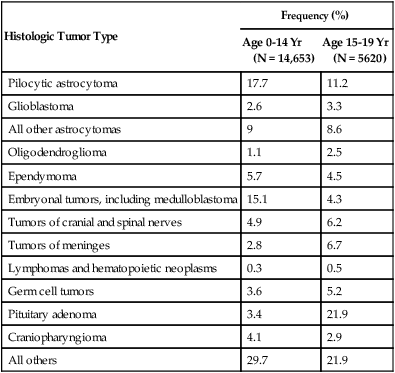
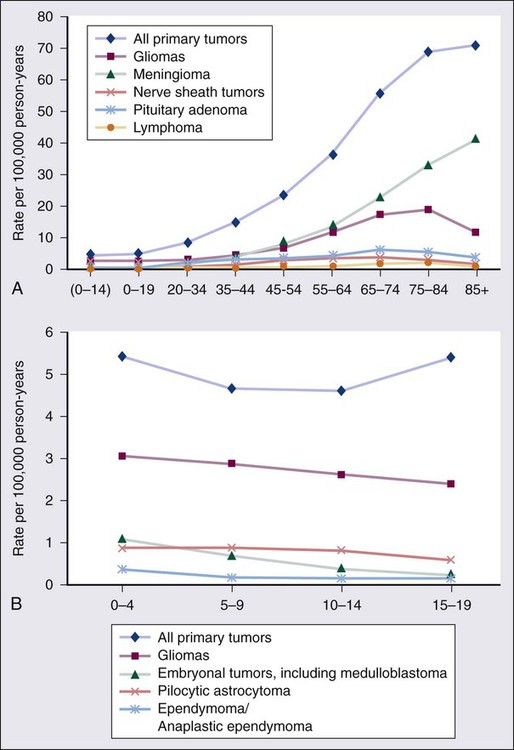
66 Jay F. Dorsey, Andrew B. Hollander, Michelle Alonso-Basanta, Lukasz Macyszyn, Leif-Erik Bohman, Kevin D. Judy, Amit Maity, John Y.K. Lee, Robert A. Lustig, Peter C. Phillips and Amy A. Pruitt • An estimated 66,290 new primary tumors of the central nervous system (CNS) will be diagnosed in the United States in 2012, of which approximately one third will be malignant. • An estimated 4200 new childhood (ages 0 to 19 years) primary benign and malignant CNS tumors will be diagnosed in 2012. Brain tumors are the second most common malignancy among children, second only to leukemias. • Radiation exposure is the best established risk factor for brain tumors. Hereditary syndromes including neurofibromatosis type 1 and 2, tuberous sclerosis, von Hippel–Lindau, and Li-Fraumeni are associated with higher risk of brain tumors. • Taking all age groups into account, histologic types of CNS tumors include meningiomas (35%), glioblastomas (16%), other astrocytomas (7%), tumors of cranial and paraspinal nerves ( 9%), tumors of the sellar region (14%), oligodendrogliomas (2%), ependymomas (2%), and embryonal tumors including medulloblastomas (1%). • Among children 14 years or younger, histologic tumor types include pilocytic astrocytomas (17%), glioblastomas (3%), other astrocytomas (9%), ependymomas (6%), oligodendrogliomas (1%), embryonal tumors including medulloblastomas (15%), craniopharyngiomas (4%), and germ cell tumors (4%). • Most brain tumors are supratentorial; notable exceptions include brainstem gliomas, cerebellar pilocytic astrocytomas, medulloblastomas, and ependymomas that involve the posterior fossa. • Glioblastoma (World Health Organization grade IV astrocytoma) and brainstem gliomas in children carry the poorest prognosis. Pilocytic astrocytomas carry the best prognosis. • General signs and symptoms from mass effect, increased intracranial pressure, edema, or shift or destruction of surrounding brain tissue may include changes in personality and cognitive function, headaches, nausea, vomiting, seizures, and papilledema. • Focal signs and symptoms may include focal seizures, visual changes, speech abnormalities, gait abnormalities, and cranial nerve deficits. • Posterior fossa tumors often compress the fourth ventricle, causing hydrocephalus, and frequently manifest with ataxia and intractable nausea and vomiting. • Brainstem gliomas often manifest with a combination of cranial nerve palsies and “long tract” signs such as hemianesthesia or hemiparesis coupled with ataxia in cases with cerebellar involvement. • Pineal region tumors (germ cell tumors, pineocytomas, and pineoblastomas, as well as gliomas of this region) may compress the aqueduct of Sylvius, causing hydrocephalus. Compression of the pretectal area produces Parinaud syndrome, with paralysis of upgaze, ptosis, and loss of pupillary light reflexes, along with retraction-convergence nystagmus. • Magnetic resonance imaging with gadolinium contrast is the most sensitive technique. • Computed tomography scanning is good for visualizing intratumoral calcifications and bone erosion but poor at visualizing the posterior fossa. • Positron emission tomography and advanced magnetic resonance imaging techniques may help discriminate between tumor recurrence and radiation necrosis or pseudoprogression. • Magnetic resonance spectroscopy may help distinguish a high-grade tumor from a low-grade tumor or radiation necrosis. • For most brain tumors, tissue diagnosis is required (an exception may be selected brainstem gliomas). • Treatment for brain tumors is highly dependent on histologic type. For many tumors (e.g., gliomas, meningiomas, primitive neuroectodermal tumors [PNETs], and ependymomas), maximal surgical resection that is safely feasible is the primary treatment. • For some tumors (e.g., glioblastomas, PNETs, and germ cell tumors), radiation therapy is an essential adjunct treatment after surgery. • For some tumors (e.g., acoustic neuromas and glomus tumors), either irradiation or surgery can offer successful control; the decision between the two is based on assessment of adverse effects. • Chemotherapy is assuming an increasingly important role in the management of many brain tumors (e.g., glioblastomas, germ cell tumors, anaplastic oligodendrogliomas, PNETs, and CNS lymphomas). The World Health Organization (WHO) classification of central nervous system (CNS) tumors is based on tumor histology and cellular origin and was established to provide a common classification and grading of human CNS tumors that is accepted and used worldwide. The WHO divides brain tumors into classes: neuroepithelial tumors, tumors of the cranial and paraspinal nerves, tumors of the meninges, lymphomas and hematopoietic neoplasms, and germ cell tumors and tumors of the sellar region.1 The different histologic types of CNS tumors are shown in Table 66-1. Meningiomas, glioblastomas, and astrocytomas constitute more than half of all CNS tumors. The frequency of different histologic tumor types varies with age, as shown in Figure 66-1. The incidence of all brain tumors is highest in the 75- to 84-year-old group. The incidence of meningiomas increases with increasing age, whereas for gliomas and pituitary adenomas, the incidence increases with age but then declines at the highest age category (see Fig. 66-1, A). Certain histologic types, such as germ cell tumors, medulloblastomas, and pilocytic astrocytomas, are far more common in children than in adults (Table 66-2; See Fig. 66-1, B). Table 66-1 Primary Central Nervous System Tumors: Distribution by Histologic Type CNS, Central nervous system; WHO, World Health Organization. *Frequency among all patients with primary brain and CNS tumors (N = 295, 986). Other neoplasms without frequency listed together account for the remaining 6.7%. †Frequency among young adults (20 to 34 years of age) with primary brain and CNS tumors (N = 25,250). Other neoplasms without frequency listed together account for the remaining 4.6%. Data from WHO 2007 Classification of Tumors of the Central Nervous System and from Central Brain Tumor Registry of the United States (CBTRUS), 2004-2008. Table 66-2 Primary Central Nervous System Tumors of Childhood: Distribution by Histologic Type Data from Central Brain Tumor Registry of the United States (CBTRUS 2012), 2004-2008. An estimated 66,290 new cases of primary CNS tumors were expected to be diagnosed in the United States in 2012.2 Approximately 22,910 of these tumors were expected to be malignant, representing 1.4% of all primary malignant cancers diagnosed in the United States.3 Malignant CNS tumors were expected to cause approximately 13,700 deaths in 2012.3 On the basis of data provided by the Surveillance, Epidemiology, and End Results (SEER) Program, Deorah and colleagues4 found that the incidence of brain cancer increased until 1987, when the annual percentage of change reversed direction. Elderly persons experienced an increase in brain cancer until 1985, but their rates were stable thereafter. Overall, however, the incidence of glioblastoma has been increasing, with survival only modestly improved during the past decade.5 Ionizing radiation is one of the few factors shown to have a strong association with the development of brain tumors. Exposure to ionizing radiation represents the most important exogenous risk factor for childhood brain tumors. Prenatal diagnostic x-ray exposure increases the risk of childhood brain tumors,6 and various reports describe the occurrence of gliomas, meningiomas, and other brain tumors in children who received radiation therapy to the head for tinea capitis and for prior malignancies.7–10 A dose of 1 to 2 Gy of radiation, which was used to treat tinea capitis in Israeli children, was associated with an increased risk of the development of brain tumors—specifically, meningiomas, gliomas, and nerve sheath tumors.11 A dose-response correlation in the induction of brain tumors was seen, with a relative risk of 3.0 at a dose of 1 Gy. Tumors developed at least 6 years after irradiation, with a mean interval greater than 15 years. Even lower doses of radiation delivered with radium-226 that were used to treat hemangiomas in Swedish infants (with a mean dose to the brain of 7 cGy) were found to be associated with an excess risk of intracranial tumors, including pituitary adenomas, gliomas, meningiomas, and nerve sheath tumors.12 A large amount of data has been accumulated on the incidence of brain tumors in patients who received cranial irradiation for the treatment of acute lymphoblastic leukemia (ALL). The estimated cumulative risk of secondary malignant brain tumors after childhood ALL therapy is 0.5% at 10 years after completion of therapy.13 In a study from St. Jude Children’s Research Hospital, the actuarial 20-year probability of developing a brain tumor in these patients was 1.4%.14 The probability of developing a high-grade glioma was greater in children younger than 5 years of age at diagnosis than in those 6 years of age or older (1.08% vs. 0.45%; P = 0.045). An apparent dose-response correlation was observed: the 20-year risk of developing a brain tumor was 3.2% in patients who received greater than 30 Gy, versus 1.03% in patients who received 21 Gy or less (P = .015). No CNS malignancies were seen in patients who did not receive cranial irradiation. The latency between irradiation and the diagnosis of a brain tumor ranged from 5.9 to 29 years (median, 12.6) but was longer for meningiomas (median, 19 years) than for high-grade gliomas (median, 9.1 years). Very similar results regarding the frequency of brain tumors, latency, and dependence on prior cranial irradiation were seen in studies from the German Berlin-Frankfurt-Munster group15 and the Children’s Cancer Study Group.16 The types of brain tumors that have been reported in these series include gliomas, meningiomas, and medulloblastomas. Patients who received cranial irradiation for ALL also often received intrathecal chemotherapy. It has been suggested that cranial irradiation and intrathecal chemotherapy may work synergistically to increase the incidence of glial tumors.17,18 Viruses can induce brain tumors in animals in the experimental setting; however, no conclusive data point to viruses as a cause of brain tumors in humans (reviewed by Berleur and Cordier19). The relationship of human cytomegalovirus (HCMV) and gliomas remains controversial. The HCMV and gliomas symposium held in April 2011 resulted in consensus that sufficient evidence exists to conclude that HCMV viral gene expression exists in most, if not all, malignant gliomas and that HCMV could influence the phenotype of malignant gliomas by interacting with signaling pathways.20 Other authors who have used the Cytomegalovirus Seroprevalence in the United States data from the National Health and Nutrition Examination Surveys, 1988-2004, have concluded that a possible CMV-glioblastoma association could not be corroborated with CMV seropositivity rates.21 Also lacking is conclusive evidence that occupational exposure to industrial chemicals leads to the development of brain tumors, although a number of studies have suggested such a link (reviewed by Wrensch and coworkers22). Some of the chemicals that can induce brain tumors in laboratory animals, such as polycyclic aromatic hydrocarbons, can do so only when administered by direct contact or transplacentally, but not by inhalation or dermal contact; the latter two modes of exposure are more relevant in the occupational setting. Specific chemicals that have been examined include cosmetics containing N-nitroso compounds, organic solvents, chemicals used in the manufacture of synthetic rubber, formaldehyde, phenols, polycyclic aromatic compounds, polyvinyl chloride, and pesticides. Although many chemicals can induce brain tumors in laboratory animals, no definitive associations have been found in humans. For example, N-nitroso compounds, which commonly are present in foods, are known to be neurocarcinogenic in animals. Oxidants in the environment can cause DNA damage, so it has been hypothesized that antioxidants, such as vitamin E, found in certain foods may protect against the development of cancers. Epidemiologic studies, however, have provided mixed support for the idea that the intake of N-nitroso compounds, antioxidants, or specific nutrients in foods can influence the risk of developing brain tumors.19 Vinyl chloride can induce brain tumors in rats; however, a recent review found that the association in humans is inconclusive.23 Recently a great deal of interest has emerged in a possible association between the use of cell phones and the risk of brain tumors. In a case-control study, Inskip and coworkers were unable to show a correlation between the duration of cell phone use and the development of gliomas, meningiomas, and acoustic neuromas.24 Other large case-control studies also have failed to find any association between cell phone use and the risk of developing brain tumors.27–27 A metaanalysis of 9 case-control studies revealed no increased risk in analog or digital cellular phone users.28 Nevertheless, some still claim that there is a link between cell phone use and brain tumors.29 Additionally, the International Agency for Research on Cancer (IARC), a WHO agency, has classified radiofrequency electromagnetic fields as “possibly carcinogenic to humans.”30 Other factors that have been analyzed for their possible relationship to the development of brain tumors include a history of head trauma and injury, drugs and medications, allergies, seizures, smoking and alcohol consumption, and exposure to power-frequency electromagnetic fields. None of these factors, however, has been conclusively shown to be important (reviewed by Wrensch and associates22). Most brain tumors represent sporadic cases; however, familial clustering has been noted. It is estimated that hereditary syndromes account for 2% of childhood brain tumors, although this may be an underestimate because hereditary syndromes may be undiagnosed in a number of cases.31 Some hereditary syndromes known to be associated with brain tumors are listed in Table 66-3 (reviewed by Kimmelman and Liang32). Some of the associations are extremely strong. Nearly 70% of all optic pathway gliomas occur in patients with neurofibromatosis type 1 (NF1), and acoustic neuromas commonly occur in patients with NF2. In addition, hereditary immunosuppression disorders such as Wiskott-Aldrich syndrome, as well as treatment-associated immunosuppression as in organ-transplant recipients, or exogenous immunosuppression as in human immunodeficiency virus (HIV) infection, are known to be associated with an increased risk of primary CNS lymphoma. Table 66-3 Hereditary Syndromes Associated with Brain Tumors Normal cells rely on growth factors secreted in their local environment to stimulate their growth. However, many CNS tumors have developed the ability to express their own growth factors along with the respective receptors, resulting in an autocrine loop that allows for self-stimulation.33 Platelet-derived growth factor receptor–α (PDGFR-α), for example, is overexpressed in all grades of astrocytomas, but only higher-grade tumors overexpress the ligands PDGFA and PDGFB.34 Insulin-like growth factors and their receptors both are expressed in brain tumors, including gliomas and meningiomas.35 The epidermal growth factor receptor (EGFR) is amplified or overexpressed in 50% of glioblastomas,33 and expression of transforming growth factor–α (TGF-α), a ligand that binds to this receptor, is increased in many gliomas.36,37 Both scatter factor (also known as hepatocyte growth factor) and its receptor c-Met are expressed in gliomas, with the highest level of expression seen in the most malignant tumors.38 As a result of increasing expression of receptors and ligands, increased signaling occurs in many brain tumors, resulting in activation of many different pathways that are important in proliferation (reviewed by Rao and James36). The best studied of these pathways is the microtubule-associated protein kinase (MAPK) pathway, which involves Ras and Raf. Another pathway that has attracted much attention recently is the phosphoinositide-3 (PI3) kinase pathway, which leads to activation of Akt. Mutation of PTEN, which occurs in 30% to 40% of glioblastomas, also can lead to increased Akt activation.39 Ras activation commonly is seen in human astrocytomas and neurofibromas despite the fact that these tumors rarely contain Ras mutations. In astrocytomas, Ras activation probably occurs through activation of growth factor receptors such as EGFR and PDGFR.40 Other mechanisms of activation of aberrant G proteins have been identified in other CNS tumors (reviewed by Woods and co-workers41). In NF1, loss of expression of neurofibromin, an inactivator of Ras, is seen (see Table 66-3). This loss of neurofibromin leads to the increased Ras activation seen in NF1-associated astrocytomas. Pituitary adenomas often show activation of the α subunit of the large heterotrimeric Gs protein, resulting in mitogenic signaling. The Wnt pathway has been shown to play a role in glioma tumorigenesis. Investigators have shown that the Wnt-specific secretory protein Evi is overexpressed in astrocytomas.42 Furthermore, the same researchers showed that the depletion of this protein in glioma cells leads to decreased cell proliferation and apoptosis. Additionally, silencing of Evi in glioma cells leads to reduced cell migration and the ability to form tumors in vivo.42 Involvement of Wnt/β-catenin signaling in gliomas has been reviewed by Zhang et al.43 Many brain tumors, particularly gliomas, display an invasive phenotype with infiltration of tumor cells into surrounding tissues, making a cure very difficult to achieve. In fact, tumor recurrence was reported in one case even after the drastic measure of taking out the entire hemisphere in which a glioma was located.44 The process of invasion involves several steps: detachment of the invading cell from the primary tumor mass, adhesion to extracellular matrix (ECM), degradation of ECM, and cell motility and contractility.45 The details of the mechanisms of glioma invasion are only beginning to be understood. Numerous molecules associated with invasion have been found to be upregulated in gliomas, including tenascin-C, secreted protein acidic and rich in cysteine (SPARC), various integrins, and matrix metalloproteinases (reviewed by Demuth and Berens46). Some controversy exists regarding deregulated Rho guanosine triphosphatase (GTPase) signaling in brain tumors. For example, although RhoA and RhoB are reduced in some astrocytic tumors, some studies report Rho-dependent lysophosphatidic acid–induced migration in glioma cells. Expression of another member of the Rho subfamily, Rac1, has been found to be reduced in astrocytic tumors; additionally, this protein has been shown to be overexpressed and to have induced invasion in medulloblastoma tumors. The role of Rho GTPases has been reviewed by Khalil and El-Sibai.47 For a tumor to grow beyond a certain size, it must develop a blood supply. The process of angiogenesis is described in detail in Chapter 8. The vasculature of normal brain, which is composed of endothelial cells, pericytes, and astrocytes, is highly specialized. Together these cells form the blood-brain barrier, which selectively restricts exchange of molecules between the intra- and extracerebral circulatory systems. As primary brain tumors or brain metastases grow, the integrity of the blood-brain barrier is compromised.48–51 A number of growth factors are known to be important in angiogenesis. The most prominent of these is vascular endothelial growth factor (VEGF), which is overexpressed in many brain tumors. In one study, increasing VEGF expression correlated with increasing malignant grade in astrocytomas, oligodendrogliomas, and ependymomas.52 In this study it also was found that increased expression of the VEGF receptors Flt-1 and KDR in tumor vasculature correlated with increasing VEGF expression and malignant grade. Hypoxia and acidosis have been shown to independently regulate VEGF transcription in brain tumors in vitro and in vivo.53 Additionally, both tumor suppressors and oncogenes, as well as hormones, cytokines, and other signaling molecules, have been shown to regulate VEGF expression.51,54–57 Growth factors other than VEGF that may play a role in angiogenesis in gliomas include members of the TGF-β family, PDGF, placenta growth factor, basic fibroblast growth factor, and scatter factor/hepatocyte scatter factor.58 Angiogenesis can also be negatively regulated by factors such as thrombospondins 1 and 2 (TSP1 and TSP2). TSP1 is positively regulated by p53, and thus loss of p53, which commonly occurs in gliomas, can lead to decreased TSP1 expression and increased angiogenesis.58 Angiogenesis in brain tumors has been reviewed by Jain et al.51 Angiogenesis is particularly prominent within glioblastomas. One of the characteristic features of these tumors is endothelial proliferation and neovascularization. A variety of growth factors that can increase angiogenesis are expressed by glioblastomas, with the foremost being VEGF. VEGF, also known as vascular permeability factor, is a potent inducer of capillary permeability. The high levels of VEGF expression in glioblastomas may be responsible for the edema associated with these tumors. Some evidence indicates that genetic changes common to glioblastomas, such as EGFR activation and PTEN mutation, may contribute to high levels of VEGF expression, perhaps through activation of the PI3 kinase pathway.61–61 Despite expressing high levels of VEGF, glioblastomas contain significant regions of hypoxia, which may be a cause of treatment resistance. Hypoxia has been shown to be present in malignant gliomas by both polarographic needle electrode measurement62,63 and binding of the 2-nitroimidazole EF5.64 Hypoxia, as measured by binding of EF5, has been shown to correlate with more rapid tumor recurrence.64 Evidence indicates that hypoxia is involved in many aspects of tumor growth, including induction of angiogenesis, increased invasion and metastases, resistance to chemotherapy, resistance to radiotherapy, increased brain tumor stem cell population, and stem cell genetic instability.65 The presence of hypoxia in glioblastomas is consistent with the histologic features commonly seen in these tumors, including the presence of pseudopalisading necrosis and proliferative blood vessels. It has been proposed that pseudopalisades seen within glioblastomas represent a wave of tumor cells actively migrating away from central hypoxia that arises as a result of vasoocclusion and intravascular thrombosis.66 It may seem counterintuitive that hypoxia can persist in the presence of high VEGF levels and robust neovascularization; however, the explanation may be that although hypoxia may stimulate VEGF and the formation of new vessels, many of these vessels are nonfunctional and do not transport oxygen well. Identification of glioblastoma stemlike cells has proved to be challenging. Early studies showed differences between CD133+ and CD133− glioma cells, including differences in tumor-forming capacity in xenografts.67 However, other studies have shown that CD133− stemlike cells may also be capable of forming tumors, although these two cell populations exhibit distinct molecular profiles and growth characteristics.68
Cancer of the Central Nervous System
Introduction
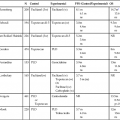
WHO 2007 Classification of Primary CNS Tumors
Frequency (%) of Primary CNS Tumors by Histology
Grade
In All Age Groups*
Among Young Adults†
TUMORS OF NEUROEPITHELIAL TISSUE
33.0
37.7
ASTROCYTIC TUMORS
6.8
12.7
Pilocytic astrocytoma
I
3
Diffuse astrocytoma
II
Anaplastic astrocytoma
III
Glioblastoma
IV
16.3
4.7
OLIGODENDROGLIAL TUMORS
1.9
4.8
Oligodendroglioma
II
Anaplastic oligodendroglioma
III
OLIGOASTROCYTIC TUMORS
Oligoastrocytoma
II
Anaplastic oligoastrocytoma
III
EPENDYMAL TUMORS
Ependymoma
II
1.8
3.8
Anaplastic ependymoma
III
NEURONAL AND MIXED NEURONAL-GLIAL TUMORS
Gangliocytoma
I
Ganglioglioma
I
Anaplastic ganglioglioma
III
Paraganglioma
I
TUMORS OF THE PINEAL REGION
Pineocytoma
I
Pineoblastoma
IV
EMBRYONAL TUMORS
1.1
1.9
Medulloblastoma
IV
CNS primitive neuroectodermal tumor
IV
Atypical teratoid/rhabdoid tumor
IV
TUMORS OF CRANIAL AND PARASPINAL NERVES
8.5
9.1
Schwannoma (neurilemmoma, neurinoma)
I
Malignant peripheral nerve sheath tumor
II, III, IV
TUMORS OF THE MENINGES
TUMORS OF MENINGIOTHELIAL CELLS
Meningioma
I
34.7
13.7
Atypical
II
Anaplastic (malignant)
III
OTHER NEOPLASMS RELATED TO THE MENINGES
Hemangioblastoma/hemangioma
I
3.5
LYMPHOMAS AND HEMATOPOIETIC NEOPLASMS
2.3
1.5
GERM CELL TUMORS
0.5
1.3
TUMORS OF THE SELLAR REGION
14.4
28.6
Craniopharyngioma
I
0.9
1.5
Pituicytoma
I
13.5
27.1
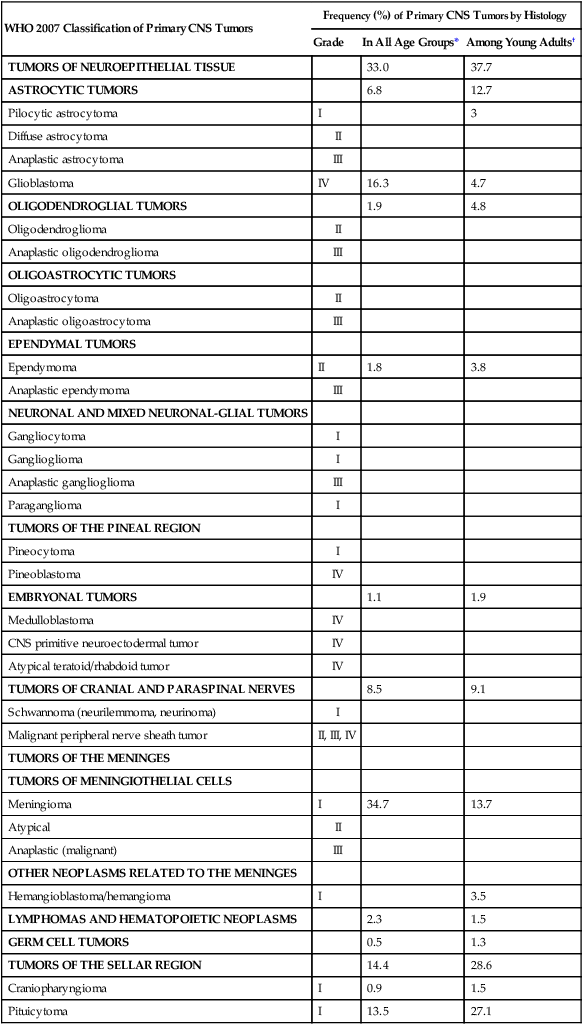
Histologic Tumor Type
Frequency (%)
Age 0-14 Yr
(N = 14,653)
Age 15-19 Yr
(N = 5620)
Pilocytic astrocytoma
17.7
11.2
Glioblastoma
2.6
3.3
All other astrocytomas
9
8.6
Oligodendroglioma
1.1
2.5
Ependymoma
5.7
4.5
Embryonal tumors, including medulloblastoma
15.1
4.3
Tumors of cranial and spinal nerves
4.9
6.2
Tumors of meninges
2.8
6.7
Lymphomas and hematopoietic neoplasms
0.3
0.5
Germ cell tumors
3.6
5.2
Pituitary adenoma
3.4
21.9
Craniopharyngioma
4.1
2.9
All others
29.7
21.9
Epidemiology
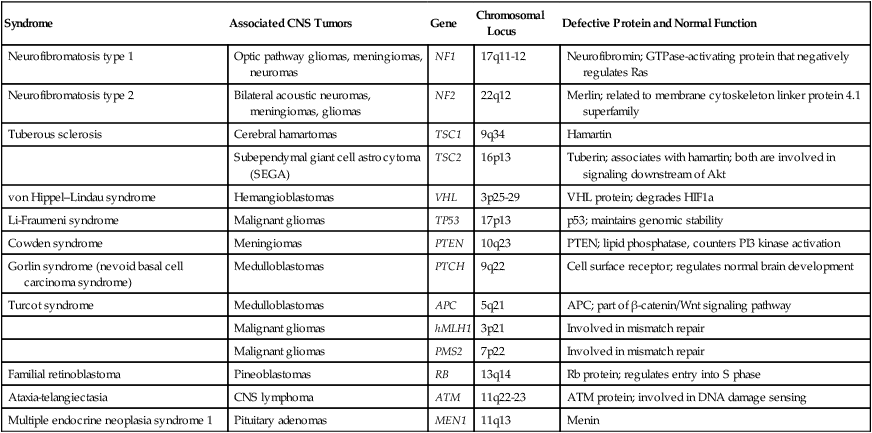
Syndrome
Associated CNS Tumors
Gene
Chromosomal Locus
Defective Protein and Normal Function
Neurofibromatosis type 1
Optic pathway gliomas, meningiomas, neuromas
NF1
17q11-12
Neurofibromin; GTPase-activating protein that negatively regulates Ras
Neurofibromatosis type 2
Bilateral acoustic neuromas, meningiomas, gliomas
NF2
22q12
Merlin; related to membrane cytoskeleton linker protein 4.1 superfamily
Tuberous sclerosis
Cerebral hamartomas
TSC1
9q34
Hamartin
Subependymal giant cell astrocytoma (SEGA)
TSC2
16p13
Tuberin; associates with hamartin; both are involved in signaling downstream of Akt
von Hippel–Lindau syndrome
Hemangioblastomas
VHL
3p25-29
VHL protein; degrades HIF1a
Li-Fraumeni syndrome
Malignant gliomas
TP53
17p13
p53; maintains genomic stability
Cowden syndrome
Meningiomas
PTEN
10q23
PTEN; lipid phosphatase, counters PI3 kinase activation
Gorlin syndrome (nevoid basal cell carcinoma syndrome)
Medulloblastomas
PTCH
9q22
Cell surface receptor; regulates normal brain development
Turcot syndrome
Medulloblastomas
APC
5q21
APC; part of β-catenin/Wnt signaling pathway
Malignant gliomas
hMLH1
3p21
Involved in mismatch repair
Malignant gliomas
PMS2
7p22
Involved in mismatch repair
Familial retinoblastoma
Pineoblastomas
RB
13q14
Rb protein; regulates entry into S phase
Ataxia-telangiectasia
CNS lymphoma
ATM
11q22-23
ATM protein; involved in DNA damage sensing
Multiple endocrine neoplasia syndrome 1
Pituitary adenomas
MEN1
11q13
Menin
Tumor Biology
Cell Proliferation
Invasion
Angiogenesis and Hypoxia
Stem Cells
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cancer of the Central Nervous System