58 Cancer of the Breast
Breast cancer accounts for 26% of all malignancies in women and is the second most common cause of cancer death in women.1 The last decade has witnessed major changes in our understanding of the biology and heterogeneity of the disease. This knowledge has resulted in the development of new therapies and treatment strategies with an emphasis on tailored therapy (i.e., that which is designed specifically for the individual patient based on tumor biology and extent of disease). This approach has been implemented in the surgical management of breast cancer, with sentinel node biopsy replacing axillary dissection as the initial staging procedure and skin-sparing or total skin-sparing mastectomy as an alternative to total mastectomy. Systemic therapies targeting molecular pathways or receptors that have proven effective in the metastatic setting are currently being evaluated in the adjuvant and neoadjuvant setting. Therapeutic interventions to manipulate the tumor stroma or microenvironment include the use of antiangiogenesis factors. Decisions for adjuvant chemotherapy may now be based on gene expression profiles and molecular subtypes of the tumor. Pathologic complete response to neoadjuvant chemotherapy has become an intermediate surrogate for disease-free and overall survival, and factors predicting for a complete pathologic response have become the focus of a number of clinical trials. The impact of systemic therapy on local control has received increasing attention with the recent meta-analysis of randomized trials demonstrating that one breast cancer death will be avoided for every four local recurrences prevented.
Breast Anatomy and Routes of Spread
The lymphatic channels are present in the subareolar skin and follow the duct and lobular complexes and most frequently drain into the lymph node chains located in the axillary basin. Breast lymphatics can also directly communicate with the infraclavicular/supraclavicular or internal mammary lymph node chains. Intramammary nodes are located within the breast parenchyma and can contain the metastatic tumor from the primary site. The axillary nodes are divided into three levels (I-III) based on their relationship with the pectoralis minor muscle (Fig. 58-1). Level I is located caudal and lateral, level II nodes are beneath the muscle, and level III (infraclavicular) nodes are located cranial and medial to the pectoralis minor. Rotter nodes or intrapectoral nodes are sandwiched between the pectoralis major and minor muscles. Orderly spread of tumor cells from the primary breast tumor most commonly starts at the level I axillary nodes and then proceed to level II and level III lymph nodes. “Skip” metastases that defy this order can occur but are less likely and a standard axillary dissection for sentinel positive disease requires pathologic analysis of level I and level II axillary nodes.
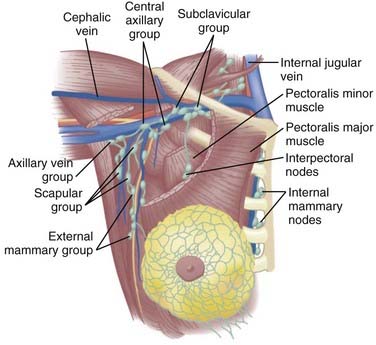
FIGURE 58-1 • Schematic representation of the regional nodes for breast cancer.
(From Donegan WL, Spratt JS: Cancer of the breast, ed 3, Philadelphia, 1988, Saunders, p 19.)
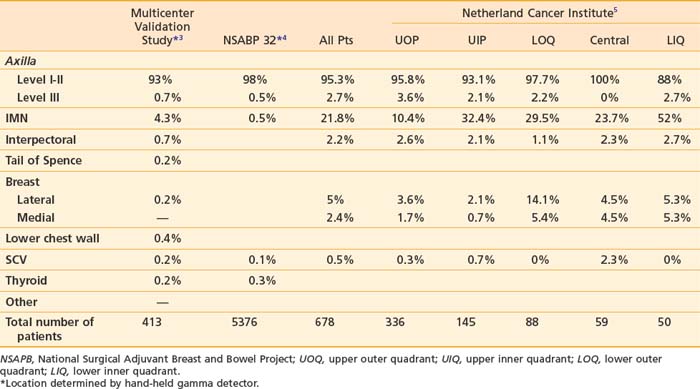
The supraclavicular lymph nodes are located within the space defined by the omohyoid muscle and tendon (lateral and superior borders), the internal jugular vein (medial border), and the clavicle and subclavian vein (inferior border).2 The internal mammary lymph node chain is encased within the endothoracic fascia in the parasternal space and runs alongside the corresponding artery and vein. The nodes located in the first through third intercostal spaces are the most common lymphoscintigraphic sites of drainage. Table 58-1 presents the location of the sentinel nodes as identified on lymphoscintigrams following peritumoral or intratumoral injection of technetium-99 sulfur colloid in women with early stage breast cancer. In the NSABP B-32 trial and the multicenter validation study, lymphoscintigrams were not required and the location of the sentinel nodes was determined by a hand-held gamma detector.3,4 This may account for the lower reported incidence of internal mammary nodes in these two studies. Overall, level I-II axillary nodes were the most common location for sentinel nodes regardless of tumor location. Internal mammary nodes were identified in less than 5% of women when lymphoscintigrams were not performed. Internal mammary nodes are not identified if the injection is intradermal.6 Estourgie et al.5 reported localization to the internal mammary node (IMN) node in 21.8% of all patients with the use of lymphoscintigrams. Inner quadrant tumors had a higher incidence of hot spots in the IMN nodes with the highest frequency in tumors in the lower inner quadrant. Upper quadrant lesions are more commonly localized to the second, third, and fourth interspaces while central and lower quadrant lesions are more commonly localized to the second, third, fourth, and fifth interspaces. Drainage to the supraclavicular nodes occurred in less than 1% of patients except for those with a central primary tumor. It should be noted that localization to a nodal region does not mean nodal positively. Biopsy of hot internal mammary nodes has demonstrated nodal positively in 8% to 27% of women.7
Epidemiology
Breast cancer is a major public health problem throughout the world. In 2007,8 it was estimated that 1.3 million women were diagnosed with new cases of breast cancer and 465,000 deaths from breast cancer were expected. Breast cancer remains the most frequently diagnosed cause of cancer death in women worldwide, only slightly surpassed by cervical cancer in economically underdeveloped countries. In the last 25 years, overall breast cancer rates have risen, including in Africa and Asia, continents with very low rates of breast cancer. The highest incidence of breast cancer occurs in North America, Northern and Western Europe, and Australia.8–10 The San Francisco Bay area has the highest rate of breast cancer in the United States.11 Currently a woman living in the United States has a 12% or a 1 in 8 lifetime risk of developing breast cancer. In 2009 in the United States, an estimated 192,370 new cases of invasive breast cancer will be diagnosed among women along with an estimated 62,280 additional cases of in situ breast cancer.1 Approximately 40,930 women are expected to die from breast cancer. About 1910 cases of breast cancer are expected to occur among men with 450 deaths.
From 1980 to 2001, breast cancer incidence rates initially increased rapidly followed by a slow increase after 1987. From 2001 through 2004, the incidence rates dropped 3.5% per year. The increase in the 1980s is largely attributed to the introduction and routine use of screening mammography and is reflected by the shift in the increase in detection of smaller previously nonpalpable breast tumors (2 cm) and a decrease in tumors of 3 cm by nearly a third.12 The increase has also been attributed to the popular use of hormone replacement therapy (HRT) and lifestyle choices that changed reproductive patterns. After 2000, the incidence rates began to drop in the United States, due largely in part to the declining use of HRT following the publication of the Women’s Health Initiative randomized trial in 200213–16 and a measurable reduction in the number of annual screening mammograms.17 Among women younger than 50, incidence rates have remained unchanged since 1986. Early detection by screening mammography also brought an increased incidence of in situ breast tumors in the 1980s and 1990s, particularly in women 50 years and older, but has since leveled off in this population. However, the incidence of in situ breast cancer continues to rise in young women.18
Epidemiologic studies of breast cancer focusing on age, race, ethnicity, and geographical location have shown disparities.19 For example, although whites have a higher incidence of breast cancer than African American women, African American women have a higher incidence before the age of 40 years and a higher mortality rate at any age.9,20
Risk Factors
Aside from being female, age is the single most important breast cancer risk factor. According to the National Cancer Institute, the risk between ages 30 and 39 is 0.43% (1 in 233), 40 and 49 is 1.44% (1 in 69), 50 and 59 is 2.63% (1 in 38), and between 60 and 69 is 3.65% (1 in 27) based on probabilities for the whole population and not individual risk factors.10
A family history of breast cancer particularly in a first-degree relative is a significant risk factor, and the risk escalates with the number of relatives affected and younger age at diagnosis. This pattern suggests an inherited genetic mutation that predisposes to the development of breast cancer. Approximately 5% to 10% of breast cancer patients have a familial form of the disease.21 Many of these cases contain an alteration in the breast cancer genes, BRAC1 and BRAC2. More than 100 distinct mutations have been identified in high-risk families and it is not clear if all carry an equal cancer risk. Some populations have a higher likelihood of carrying germline mutations such as family members of Ashkenazi Jewish (Eastern European) heritage and families with multiple cases of breast and/or ovarian cancers. The estimated lifetime risk of developing a breast cancer is up to 80% (36% to 85%), with a near 40% risk of developing a contralateral breast cancer. The risk of developing an ovarian cancer is 40% in BRCA1 carriers and 20% for BRAC2 carriers.22,23 Genetic counseling should be offered to these patients including those of young age at diagnosis, two primary breast cancers (ipsilateral or contralateral) or with breast and ovarian cancer and male breast cancer.24 There are other rare familial genetic syndromes that display a predisposition to breast cancer, the most well-known are Li-Fraumeni syndrome with germline mutations in TP53 and Cowden and Bannayan-Riley-Ruvalcaba syndrome due to PTEN mutations.
The absolute risk of a contralateral breast cancer in women with a personal history is 0.5% to 1% per year or up to 10% during the 10 years following diagnosis.25,26 Biopsy-proven atypical proliferative disorders, including atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), and atypical ductal hyperplasia (ADH), may increase the risk by a range of fourfold to tenfold with a further increase in a patient with a family history.27–30
Mammographic density is a strong independent risk factor with a fourfold to sixfold increase for postmenopausal women with high breast density compared with those with least dense breasts.31–36 Breast density refers to the amount of white area (fibrous and glandular tissue) on a black (primarily fat tissue) mammogram. While methods of measurement and definitions of breast density vary among studies, women with breast densities of more than 60% to 75% have been found to have an increased risk of breast cancer.37 Higher breast density is more common in Caucasian women and younger women and decreases during menopause. Hereditary factors may account for the majority of highly dense breasts. Several studies have identified genes that influence mammographic density.38–40 Tamoxifen has been shown to decrease breast density especially during the first 18 months of treatment.41
The risk of developing breast cancer after exposure to ionizing radiation is dose and age dependent and has been demonstrated from data collected from the Japanese atomic bomb survivors and patients exposed to radiation for nonmalignant conditions, such as thymus enlargement, multiple chest fluoroscopies for tuberculosis, and mastitis examinations.42–45 Particularly susceptible are adolescents who demonstrate the greatest risk of breast carcinogenesis over a lifetime. Secondary breast cancer has been described in young women who underwent mantle irradiation for Hodgkin disease with doses ranging from 20 to 44 Gy.46 In a multi-institutional review of 1380 adolescents treated before age 16 years old, a cumulative probability of breast cancer at age 40 years was 35%. This cohort of survivors had a risk of breast cancer 75 times higher than that of the general population.47 The risk of breast cancer significantly increases 15 to 30 years after treatment for those women exposed between the ages of 10 to 30 years; however, relative risk begins to increase several years following radiation exposure.48 Current practice using lower doses of radiation and limited fields for Hodgkin disease may decrease the risk of breast cancer for these patients.49
A moderate relative risk is associated with factors which affect circulating hormone levels such as delayed childbirth, nulliparity, early or late menarche and exogenous hormones. Body mass index (BMI) or postmenopausal obesity, has clearly been associated with breast cancer risk likely due to higher estradiol levels associated with aromatase in adipose tissue, which converts androgens to estradiol. A pooled analysis of prospective studies demonstrated a 30% higher risk in postmenopausal woman with a BMI more than 31 kg/m2 compared with a BMI of less or equal to 20 kg/m2.50 Weight gain at menopause is associated with increased risk; however, weight loss with maintenance is associated with a substantial lowering of breast cancer risk.51
Alcohol consumption increases the risk of breast cancer. In the Oxford meta-analysis of 53 epidemiologic studies, 58,515 patients with breast cancer and 95,067 women without breast cancer demonstrated that two drinks a day (defined as 24 g of alcohol) can increase breast cancer risk by 21%.52 The relative risk of breast cancer was dose-dependent and increased with daily amount. Another analysis of 184,418 postmenopausal women showed that moderate intake of one to two drinks a day can increase risk by about 32% and three or more by 51%.53
Two commonly used models to estimate an individual’s breast cancer risk are the Gail and Claus models which heavily weigh family history with a combination of other risk factors.54,55 A breast cancer risk assessment tool is available on the National Cancer Institute website based on the Gail model (cancer.gov/bcrisktool). Other decision models have been developed to estimate the likelihood of a BRCA mutation include the BRCAPRO.56,57 and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA).58
Clinical Presentation and Detection
The most frequent presentation of early stage breast cancer is an asymptomatic, nonpalpable mass, which is detected as an abnormality on screening mammogram. The most common physical sign is a nonpainful mobile mass.59 A detailed physical examination includes evaluation of the ipsilateral and contralateral breast tissue and regional lymph nodes (bilateral axillae, supraclavicular, infraclavicular, anterior cervical/neck, and submental and submandibular lymph nodes chains). The treatment approach is determined, in part, by the clinical presentation such as tumor size, location, and skin involvement. A large tumor can be a contraindication to breast conservation; inner quadrant tumors require lymphoscintigraphy to identify sentinel nodes located in the internal mammary chain.
Mammographic signs of cancer consist of two primary findings: (1) a mass with ill-defined, irregular, or spiculated edges and/or (2) irregular, pleomorphic calcifications. Further diagnostic imaging including targeted ultrasonography and mammographic magnification and compression views of all suspicious areas should be completed before a biopsy procedure. The imaging reports should include size and location of the primary tumor and a description of the findings in accordance with the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) guidelines.60 A pathologic diagnosis of cancer is necessary before proceeding to definitive surgery. Fine-needle aspiration of a palpable mass or lymph node can be performed at the time of the initial evaluation and before a core needle biopsy. A benign FNA is an incomplete evaluation if discordant with the clinical findings and must be followed with an ultrasound-guided core needle biopsy, stereotactic biopsy, or an excisional biopsy. Clinically enlarged lymph nodes are also evaluated by FNA or core needle biopsy and computerized tomography (CT)/positron emission tomography (PET). Once a breast cancer diagnosis has been made, magnetic resonance imaging (MRI) can be a diagnostic tool for assessing the extent and pattern of cancer in the breast parenchyma and detecting occult contralateral cancers.61,62
Mammography as a screening tool has been proven to reduce mortality from early stage breast cancer.63–65 Additional imaging tools, such as breast ultrasound and dynamic/contrast-enhanced MRI, have not been shown to impact mortality, and given the technical and practical limitations have no universal screening role but are useful supplemental techniques. MRI has been shown to have superior sensitivity for detecting breast cancers in high-risk populations over mammography, clinical examination, and ultrasound. The cost of higher sensitivity is the variable specificity that is lower than mammography in all studies.66
In woman with a high risk of breast cancer due to a strong family history or a BRCA mutation, routine surveillance with clinical breast examination and mammography is suboptimal compared with MRI. Six prospective nonrandomized studies have demonstrated the role of MRI screening in this high-risk population.67–72 One of these studies followed 236 women with BRCA mutations with breast examination, mammography, ultrasound, and MRI. More than twice as many cancers were detected by MRI (77%) than by mammography (36%), and the combined sensitivity of all four modalities was 95% compared with the 45% from breast examination and mammography alone.68 Similar results were obtained from a larger study, which surveyed 1909 high-risk women (15% or more cumulative lifetime breast cancer risk) with annual breast examination, mammography, and MRI. With a median follow-up of 2.9 years, 51 tumors were detected with a sensitivity of 18%, 33%, and 79%, respectively, and specificity of 98%, 95%, and 90%, respectively.67
The American Cancer Society published guidelines for the use of screening magnetic resonance imaging (MRI) as an adjunct to mammography in 2007.66 Annual screening MRI was recommended for proven carriers of a BRCA mutation, untested first-degree relatives of a proven mutation carrier and women with a lifetime risk of breast cancer at 20% as determined by risk assessment models such as BRCAPRO. It was also recommended for women with a prior history of chest radiation between the ages of 10 to 30 years and for women with Li-Fraumeni syndrome or other rare genetic syndromes and their first-degree relatives. The role of annual MRI screening in women with a 15% to 20% lifetime risk for breast cancer (i.e., those with a personal history or extremely dense breasts on mammography) was not clarified. Annual MRI screening was not recommended for women with less than 15% lifetime risk of breast cancer.
Pathology
The American Joint Committee on Cancer classifies breast cancers into the following histopathologic categories73,74 in situ cancers and invasive cancers. The in situ cancers include those that are not otherwise specified (NOS), ductal carcinoma in situ (DCIS, intraductal cancer), and Paget disease with DCIS. The invasive cancers include those that are not otherwise specified, ductal, lobular, medullary NOS, or medullary with lymphoid stroma, mucinous, tubular, papillary (predominantly micropapillary pattern), Paget with invasive cancer, inflammatory, undifferentiated, squamous cell, adenoid cystic, secretory, and cribriform.
The term lobular carcinoma in situ (LCIS) is a misnomer in that LCIS is considered a marker for an increased risk for the development of breast cancer and not a cancer in itself. LCIS was first described by Foote and Stewart in 1941.75 It is characterized by the presence of large, nonuniform in size, discohesive epithelial cells that fill the acinar spaces to varying degrees.76,77 Mastectomy specimens have demonstrated LCIS to be multicentric in 90% of cases and bilateral in 35% to 59%.76,77 LCIS is usually estrogen receptor-positive. C-erbB-2 and p53 are infrequently overexpressed.77,78 Loss of E-cadherin (CDH1), an adhesion molecule, is found in 95% of LCIS.79 LCIS, when not associated with an invasive cancer, is usually detected as an incidental finding at the time of a benign biopsy. It is found more frequently in premenopausal women with an average age of 45 years.77 The invasive cancer, which develops subsequent to a diagnosis of LCIS, is most often an invasive ductal cancer, which can occur in either breast.76,80 In the National Surgical Adjuvant Breast and Bowel Project (NSABP) randomized trial for DCIS, 182 patients were inadvertently entered with LCIS. Five percent of these women who had excision alone developed a subsequent invasive cancer in the index breast compared with 5.6% in the contralateral breast.81 The respective rates for DCIS were 9% for the index breast and 1.2% for the contralateral breast. The median follow-up was 12 years. It was of interest that all of the cancers that developed in the index breast occurred in the vicinity of the LCIS. Treatment options for LCIS in the absence of an invasive cancer range from bilateral mastectomy to excision alone. Because of the propensity for the bilateral risk, unilateral mastectomy is not appropriate. The most commonly accepted approach is excision alone with close follow-up. The role of negative resection margins for LCIS in diminishing the subsequent risk is unknown. Tamoxifen has been shown to decrease (but not eliminate) the risk of cancer following a diagnosis of LCIS and has been used as a prevention strategy. In the NSABP P-1 prevention trial, tamoxifen resulted in a 56% decrease in the risk of a cancer in women with LCIS.82,83
Pleomorphic LCIS is a variant of LCIS. The majority of reported cases of pleomorphic LCIS have occurred in association with pleomorphic invasive lobular cancer.84 Pleomorphic LCIS has been found to have a higher proliferation rate and greater percentage of p53 positivity when compared with classic LCIS and is estrogen-receptor-positive, HER-2/neu negative, and E-cadherin negative.84 Necrosis may be seen in 40%.84 Recent studies have suggested that pleomorphic LCIS is more closely related to LCIS than DCIS.84,85 The biologic course of pleomorphic LCIS is unknown. In the past many of these lesions were classified as DCIS due to the presence of necrosis and calcifications. Sneige et al.84 reported outcome in 10 cases. None of the seven cases who underwent adequate excision or simple mastectomy recurred.
Ductal carcinoma in situ by definition is carcinoma confined to the pre-existing duct system of the breast without penetration of the basement membrane by light microscopy. Pathologically, DCIS may be characterized by its architectural pattern (comedo, solid, cribriform, papillary, and micropapillary), nuclear grade (low, intermediate, high), and the presence or absence of comedonecrosis. Less common architectural patterns include apocrine, clinging, signet-cell cystic, hypersecretory, and neuroendocrine.86 It is not uncommon for more than one architectural pattern to be present in a single lesion. DCIS currently represents approximately 21% of all breast cancers and 20% to 30% of those that are mammographically detected.
The natural history of DCIS is characterized by a low risk for axillary metastases (<5%) and an extremely low breast cancer mortality (<5%). DCIS is detected as a mammographic abnormality (usually calcifications) in 80% to 90% of women with 10% to 20% having a physical finding (palpable mass, bloody nipple discharge). The extent of DCIS may be underestimated by the area of calcification on the mammogram, especially if magnification views are not obtained.87–89 In addition, pathologic examinations of mastectomy specimens in women with DCIS not infrequently reveal discontinuous extension in the duct system.90 Contiguous extension has been described in two directions: one centrally towards the nipple and one peripherally and laterally.91
This somewhat unpredictable pattern of spread contributes to the difficulty in assessing the pathologic extent of DCIS. Micropapillary and low-grade lesions tend to have a more diffuse and discontinuous pattern of spread.92 In contrast to high-grade DCIS, two thirds of low-to intermediate grade DCIS have been reported to have a multifocal disease with distances between foci of up to 1 cm.87,90 More recently, MRI has been used to evaluate the extent of DCIS.93,94 In a study of 45 patients from the University of California, San Francisco, MRI overestimated the pathologic extent of disease more than twofold in 23% and underestimated it in 9%.93 A discrepancy between the pathologic size and MRI extent was more frequently observed with the MRI patterns of regional or multiregional enhancement and in estrogen receptor-positive DCIS.
Foci of occult invasion are more often observed with high-grade DCIS, those with comedonecrosis, or with a size greater than 2.5 cm88,92,95 The estrogen receptor is positive in 90% of low-grade DCIS and 25% of high-grade lesions. Overexpression of c-erBB-2 and P53 is present in less than 20% of low-grade lesions but in two thirds of high-grade lesions.96 DCIS is considered a precursor for the development of an invasive ductal cancer in the index breast. However, it is not an obligate precursor in that not all DCIS progresses to an invasive cancer. In a study of women who had undergone a biopsy for what was interpreted at the time as benign disease and on subsequent review was found to be low-grade DCIS, 39% 15 to 42 years later developed an invasive cancer in the index breast.97,98
Pathologic classification systems have been proposed to predict the clinical behavior of DCIS in terms of ipsilateral breast tumor recurrence (IBTR), either invasive or noninvasive after breast-conserving surgery and breast cancer specific survival. A system which stratifies risk for recurrence is useful in defining the roles of radiation and mastectomy. A 1997 international consensus conference99 recommended that a number of features should be described in the pathology report including nuclear grade, necrosis, polarization, architectural pattern, margin status, size, presence of absence of calcifications, and relationship of calcifications to the DCIS. Silverstein100 proposed a classification system (the University of Southern California/Van Nuys Prognostic Index-USC-VNPI) based on clinical and pathologic features to predict biologic behavior. The scoring system has four categories (age, size, margins, and nuclear grade combined with necrosis) and within each category there are three scores (1, 2, 3). The age scores are 1 greater than or equal to 60 years, 2 = 40 to 60 years, and 3 ≤ 40 years. The size scores are 1 less than or equal to 1.5 cm, 2 = 1.6-4 cm, and 3 greater than or equal to 4 cm. The margin scores are 1 greater than or equal to 1 cm, 2 = 1 to 9 mm, and 3 less than or equal to 1 mm. The pathologic scores are 1 = low grade and no necrosis, 2 = low grade with necrosis, and 3 = high grade. Summing each of the category scores results in total scores ranging from 4 to 12. These four factors were culled from a number of factors evaluated in a multivariate analysis of prognostic factors for IBTR in patients with DCIS treated in their practice. It should be noted that their work was based on detailed clinical (mammographic and operative findings) and pathologic correlation and required processing of the entire surgical specimen with a uniform thickness of 2 to 3 mm and sequential analysis, thereby providing the most accurate assessment of size and margin status. The reproducibility of this system has been questioned by a number of investigators in retrospective and prospective studies and their detailed pathologic evaluation is not commonly used in clinical practice.101–104 At the present time, there is no one system which best predicts outcome and multiple factors should be considered.
Paget disease of the breast consists of 1% to 3% of all breast cancers.105–107 It is characterized by the presence of neoplastic cells in the epidermis of the nipple. There is no penetration of the dermal basement membrane and therefore Paget is a form of in situ cancer. HER-2/neu overexpression is common in Paget disease.108,109 Paget is frequently associated with underlying ductal carcinoma in-situ or an invasive cancer or both,105,107,110 although the process may be isolated to the nipple. Chen et al.111 reported a decrease in the overall incidence of Paget disease in the Surveillance, Epidemiology and End Results (SEER) registry from 1988-2002. However, no underlying breast cancer was identified in only 13% of the 1704 women with Paget disease. Paget disease has erythema and an eczematous scaling of the nipple skin. It may progress to crusting and frank ulceration. The prognosis of Paget disease is related to that of the underlying invasive cancer, if present.
Invasive ductal cancer is the most common invasive cancer.112 Approximately 50% of invasive ductal cancers will have an associated DCIS component. The term extensive intraductal component (EIC) was first described by Schnitt et al. from Harvard.113 By definition, the entity consists of the simultaneous presence of DCIS comprising 25% or more of the primary tumor and its presence in the normal surrounding breast tissue. The definition also includes DCIS with focal areas of invasion (i.e., microinvasive cancer). While there have been various definitions of microinvasive cancer, it is currently defined by the AJCC staging manual as foci of invasion no greater than 0.1 cm.73,74 In instances where there are multiple foci of invasion, only the largest focus of invasion should be considered. The clinical presentation of EIC positive tumors is usually the presence of mammographic calcifications without an associated mass.114 The clinical significance of an extensive intraductal component is its association with an increased risk of ipsilateral breast tumor recurrence after breast-conserving surgery with or without radiation.115–122 This observation has been explained in part by the pathologic studies of Holland et al.123 In a serial subgross and correlated mammographic examination of 217 mastectomy specimens, they correlated the presence of an extensive intraductal component in an invasive ductal cancer with the incidence of residual tumor following an excisional biopsy. EIC positive tumors were significantly more likely to have residual tumor (predominantly DCIS) and at greater distances from the primary than EIC negative tumors. At a distance of 6 cm from the edge of the invasive cancer, 21% of EIC positive tumors had residual cancer compared with 8% of EIC negative tumors. The presence of EIC has not been associated with an increased risk of chest wall recurrence after mastectomy.124 EIC positive tumors have also not been found to have a higher risk of distant metastases113 and in some series have been reported to have a better prognosis.124,125
Invasive cancers can be further classified by their histologic grade. A modification of the Scharf Richardson Bloom system is commonly used. The total score for grade is based on adding the individual scores for differentiation, mitoses, and nuclear pleomorphism with each having a score of 1 to 3 and total scores of 3 to 9. Low-grade tumors have scores of 3 to 5, intermediate grade scores of 6 to 7, and high grade of 8 to 9.126 The Nottingham grading system127 assigns scores of one to three for the percentage of tubule formation, the degree of nuclear pleomorphism and mitotic count in a defined field area. The sum of the three scores determines the final grade with three grade divisions. Histologic grade is a prognostic factor for local-regional recurrence following mastectomy128–130 and breast-conserving surgery with or without radiation131–138 and has been correlated with breast cancer specific survival.134,139
The presence of lymphovascular invasion (LVI) has been associated with an increased risk of local-regional recurrence following breast-conserving surgery with or without radiation and mastectomy, and with an overall worse prognosis, especially in axillary node-negative women.115,140–150 Colleoni et al.150 found extensive lymphovascular invasion to be associated with a significant decrease in disease-free and overall survival in axillary node-negative women.
Invasive lobular cancers represent 5% to 10% of all breast cancers and represent the second most common type of breast cancer. They arise from the lobules and are often characterized by multifocality and a higher incidence of bilaterality in some series.151,152 There are five subtypes of invasive lobular cancers (classic, tubulolobular, solid, alveolar, and pleomorphic). Classic invasive lobular cancers are characterized by the presence of small relatively uniform cells that invade the stroma in a single-file pattern with little or no desmoplastic reaction. The pleomorphic variant has a similar pattern of invasion but the cells are larger and have more nuclear variation. The different subtypes of invasive lobular cancer have been associated with different prognoses.153 The pleomorphic variant has been associated with a larger primary tumor size, a greater frequency of positive nodes and a worse prognosis.154,155 Invasive lobular cancers are usually estrogen and progesterone receptor-positive and HER-2/neu negative.156,157
Several of the more uncommon histologic subtypes of breast cancer have been associated with a very favorable prognosis. These include tubular and mucinous or colloid cancers. Axillary nodal metastases are less common and they tend to occur in older women.158–161 In contrast, invasive micropapillary carcinoma is associated with a worse prognosis due to its frequent involvement of axillary nodes and larger primary tumor size.162 Medullary carcinomas are associated with a prominent lymphocytic infiltrate and are well circumscribed grossly and microscopically. While more often high grade, they tend to be associated with a better prognosis. They occur more frequently in younger women and have been associated with BRCA1 mutation carriers.163
Guidelines for the basic elements of a pathology report for breast cancer have been established by the College of American Pathologists.164 The following is a list of information that is pertinent for the radiation oncologist to consider in the decision-making process:
| Partial Mastectomy | Size of specimen |
| DCIS (with or without an invasive component)—architectural pattern, nuclear grade, presence or absence of necrosis, calcifications in benign disease or DCIS, size and extent of DCIS, multifocality, estrogen and progesterone receptor status, HER-2/neu (investigational) | |
| Invasive cancer—size and extent, histology, grade (nuclear grade, mitotic count, tubule/papilla formation), presence or absence of LVI, perineural invasion, or extensive intraductal component, presence or absence of LCIS (pleomorphic or classic), calcifications in benign disease or invasive cancer, multifocality. | |
| Estrogen and progesterone status, HER-2/neu (IHC or FISH), invasive cancer. | |
| Margins of resection—width of negative margin for DCIS and invasive cancer (anterior, posterior, superior, inferior, lateral, medial), extent of positive margin and location. | |
| Mastectomy | Type of procedure (total mastectomy, skin-sparing, total skin-sparing [i.e., nipple preserved]) |
| Skin ellipse size and size of specimen | |
| Location of primary tumor(s) | |
| DCIS (with or without an invasive component)—architectural pattern, nuclear grade, presence or absence of necrosis, calcifications in benign disease or DCIS, size and extent of DCIS, multifocality, estrogen receptor status, HER-2/neu (investigational) | |
| Invasive cancer—size and extent, histology, grade (nuclear grade, mitotic count, tubule/papilla formation) presence or absence of LVI, perineural invasion, EIC, presence or absence of LCIS (pleomorphic or classic), calcifications in benign disease or invasive cancer, multifocality, extension to muscle | |
| Estrogen and progesterone status, HER-2/neu (IHC or FISH), invasive cancer | |
| Mastectomy margins, deep, anterior superior and anterior inferior | |
| Axillary Nodes | Number of sentinel and nonsentinel nodes |
| Number of positive nodes | |
| Method of detection of positive nodes (H+E, IHC, RT-PCR) | |
| Size of nodal metastasis | |
| Presence or absence of extracapsular extension and, if present, extent |
Staging
The current staging system for breast cancer is presented in Table 58-2, A and can be found in the seventh edition of the AJCC Cancer Staging Manual.74 The prior staging system is presented in Table 58-2, B.73 The staging system relies on primary tumor size, the presence and extent of regional node involvement, and distant metastases to define prognostic categories. The clinical classification (cTNM) is based on findings from the physical examination, imaging studies including mammography, ultrasound, MRI, CT scans, but not lymphoscintigraphy and pathologic examination of breast and/or regional node biopsies. Dimpling of the skin or nipple retraction does not imply T4 disease. Extensive imaging is not required to designate a case as M0 provided the clinical history and examination do not suggest the presence of distant metastases. The term M0 (i+) has been introduced to denote patients with circulating tumor cells in the blood or bone marrow.
Table 58-2B TNM Classification for Breast Cancer from the AJCC Staging Manual, 6th Edition
Rights were not granted to include this table in electronic media. Please refer to the printed book.
Pathologically positive nodes are classified as macrometastases, if the metastatic deposit is more than 2 mm. Micrometastases are defined as deposits more than 0.2 mm and less than 2 mm. T1N1miM0 tumors are now classified as stage IB. Isolated tumor cells are defined as clusters of 0.2 mm or not exceeding 200 cells in a single cross section of a node (pN0i+). They are usually detected by immunohistochemistry or molecular methods (pN0mol+) but can be verified by hematoxylin and eosin stains. Intramammary nodes are considered axillary nodes for the staging system and metastatic deposits in the axillary fat are classified as positive nodes. The number of positive nodes and their location further defines the staging groups (Table 58-2, B). The modifier (sn) is used for patients who have sentinel node biopsies only or when the total number of nodes removed is less than 6 nodes.
Surgical Considerations
The goal of breast-conserving surgery (BCS) is to eradicate both invasive and in situ disease and to achieve a desirable cosmetic result, which can be appreciated by both the surgeon and patient. Breast-conserving surgery has been shown to be an effective treatment when compared with mastectomy, yet BCS lacks the psychological impact and impaired body image that may accompany mastectomy.165,166 The decision to undergo BCS should be a carefully thought out plan that incorporates information from pathology and radiology and patient preference in an attempt to individualize care.
Once a patient is determined to be a candidate for BCS, attention turns to the technique for surgical excision. Localization of the tumor is performed either by palpation or placement of a wire under mammography, ultrasound, or MRI guidance. New techniques for localization include placement of a radioactive seed at the tumor, which can be identified intraoperatively using a hand-held gamma probe. However, only a few institutions have incorporated this into surgical practice.167–169 The size of excised breast tissue depends on multiple factors, which include tumor size and location, breast size, and radiographic findings. Complete excision of the tumor and any suspicious radiologic findings should be done in the most cosmetically appreciated way. Some surgeons advocate removing additional margins at the time of surgery to decrease the incidence of positive or close margins at final pathology. It is not necessary to remove the skin overlying the tumor except in cases where the skin is tethered or in intimate contact with the cancer.
Standard wound closure is a two-layer closure at the dermal/epidermal level. Generally, the cavity is left alone to fill with seroma fluid so as to maintain the shape of the breast. Recently, new techniques of wound closure have incorporated breast-flap advancement for volume replacement following large partial mastectomy resections.170–173 In an attempt to offer breast-conserving surgery, patients who had previously been destined for mastectomy are now assessed for “oncoplastic” surgery. The approach to oncoplastic surgery requires the surgical team to rethink the traditional curvilinear incisions in the upper pole and radial incisions in the lower pole, and focus on an approach that allows incorporation of fibroglandular rearrangement. These newer techniques have broadened the surgical choices for many patients who have concerns regarding mastectomy; however, there are inherent risks that cannot be overlooked.174 Further excision for compromised margins could be very difficult with this volume-replacement technique, and disruption of lymphatic channels may have consequences as it pertains to future lymphatic mapping in cases of local recurrence. Planning for radiation therapy must also be considered in terms of the identification of the primary tumor site for delineation of the boost volume. Placement of surgical clips in the tumor bed is especially important in these women. There have been no randomized controlled trials involving oncoplastic surgery and the risk of a local recurrence. Small cohorts have shown promising results for both aesthetics and local control, but careful consideration must be taken into account when planning oncoplastic techniques until better, long-term data are available to support this technique for routine use.175–177
If a patient desires BCS but has a tumor/breast ratio that initially would not be amendable to lumpectomy, there is the possibility of tumor “downstaging” with neoadjuvant therapy. Chemotherapy has been shown to be equally effective whether it is given presurgery (neoadjuvant) or postsurgery (adjuvant).178,179 The discussion of neoadjuvant therapy should be a multidisciplinary approach to ensure appropriate recommendations from medical, radiation, and surgical oncology. Magnetic resonance imaging (MRI) has become a valuable tool for predicting BCS success and is rapidly gaining wide use in this setting.180–182 The MRI images can direct the surgeon to the volume of breast tissue that should be removed in relation to response to therapy. However, mammography is equally important in assessing the extent of residual calcifications that need to be excised at the time of lumpectomy. At the start of neoadjuvant therapy, the patient should have a titanium clip placed under the core biopsy of the tumor to mark the location. The titanium clip can be used for localization in cases where a complete radiographic response is achieved.183 Clip placement has been associated with improved local control in patients receiving neoadjuvant chemotherapy and undergoing breast-conserving surgery.184 The volume of breast tissue excised after neoadjuvant chemotherapy is generally the residual nidus and not the original volume. However, all malignant appearing calcifications must be excised before radiation. Margin assessment may be more difficult in tumors that regress in a multifocal pattern.
Mastectomy
Not all patients will be candidates for BCS even after neoadjuvant therapy, and many will choose mastectomy regardless for personal reasons. Mastectomy offers an excellent oncologic treatment for breast cancer, and with new surgical techniques, the cosmetic results have greatly improved if the patient desires breast reconstruction. The most recent advance in surgical technique has been the introduction of the total skin-sparing mastectomy or nipple-sparing mastectomy.185,186 Leaving the nipple-areolar complex (NAC) intact has led to considerable improvements in cosmetic results without compromising the oncologic procedure itself. The tissue directly underneath the areola is sharply dissected and the nipple is inverted to core out all ductal tissue with an approximate 5% rate of nipple loss secondary to ischemia. Contraindications to nipple-sparing mastectomy are inability to achieve acceptable margins at the NAC or direct involvement of the nipple tissue itself. Patients can choose either autologous tissue transfer or implant reconstruction following nipple-sparing mastectomy.
It is generally accepted that immediate reconstruction after mastectomy is safe in terms of local recurrence rates and does not significantly prolong the start of adjuvant therapy.187,188 Most plastic surgeons agree that immediate reconstruction offers a better aesthetic outcome in conjunction with the psychologic benefit of awakening from surgery with an intact reconstructed breast.189,190 However, there is considerable debate surrounding immediate reconstruction for those patients who are at a higher risk of requiring postmastectomy radiation therapy (PMRT) with concern for an increased rate of complications. Numerous studies have shown that radiation increases the rate of TRAM flap complications, namely fat necrosis and flap necrosis.191,192 These complications can occur both with immediate reconstruction and delayed reconstruction. Some would argue that the immediate reconstruction with skin-sparing or nipple-sparing mastectomy leads to a superior cosmetic result and that it is beneficial in light of the potential for volume loss and fibrosis that can accompany PMRT. In an attempt to avoid these complications, but also to achieve excellent cosmesis, some have implemented a “two-stage” approach.193 If the patient is deemed high risk for PMRT, they undergo either a skin-sparing mastectomy or nipple-sparing mastectomy with placement of a subpectoral tissue expander to preserve the skin envelope. After review of the final pathology, patients who do not require PMRT can undergo immediate reconstruction, while those who require PMRT can have this treatment with the expander in place. Following radiation the patient can then undergo delayed reconstruction with the preserved skin envelope.
Axilla
Sentinel lymph node biopsy has proven to be an accurate technique for staging the axilla in breast cancer patients and is associated with less morbidity when compared with complete axillary node dissection. Several prospective trials in the United States and Europe have randomized women to sentinel node biopsy and axillary dissection versus sentinel node biopsy and axillary dissection only if the sentinel node is positive. Veronesi et al.194 reported the results of the European Institute of Oncology trial for stage I breast cancer. The false-negative rate was 8.8% and the negative predictive value was 95.4%. There were no axillary recurrences in the women who had sentinel node biopsy only. In the NSABP B-32 trial of 5611 women, sentinel nodes were identified in 97.1%, the false-negative rate was 9.8%, and the negative predictive value was 96.1%.4 When performed by experienced hands, the false-negative rate has been shown to be less than 5%, with long-term recurrence rates in sentinel node-negative patients approaching 0.3%.195
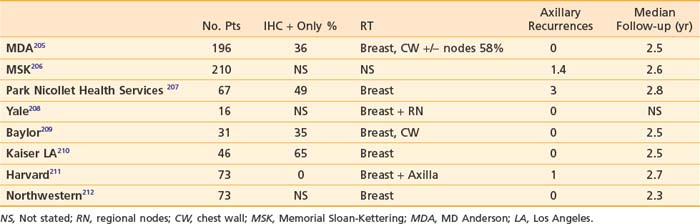
Completion axillary dissection is recommended for patients with positive sentinel nodes.196 This recommendation is based on the finding from a meta-analysis of 69 trials of sentinel node biopsy of 48% nonsentinel node positivity in women with positive sentinel nodes.195 However, the role of completion axillary dissection in women with immunohistochemical (IHC) only positive sentinel nodes or micrometastases (>0.2 mm and <2 mm) is unknown. A number of predictive models have been developed to estimate the risk of nonsentinel node positivity in patients with positive sentinel nodes. These include three nomograms197–199 and three scoring systems.200–202 Factors evaluated in these models include method of detection (frozen section, routine H+E, serial H+E, or IHC), primary tumor size, nuclear grade, multifocality, presence of lymphovascular invasion, estrogen receptor status, size of sentinel node metastasis, number of positive sentinel nodes, and number of sentinel nodes removed. It has been suggested by some investigators that women with a less than 10% risk of nonsentinel node positivity may forego completion axillary dissection.203,204 The role of axillary irradiation in patients with positive sentinel nodes who do not undergo an axillary dissection has been evaluated in eight single institution studies (Table 58-3).
It should be noted that in a significant number of patients, the sentinel node had isolated tumors cells or was IHC positive only. Overall axillary recurrence rates are 3% or less with median follow-up times of 2.5 years. Axillary recurrence appears to be low whether the low axilla is included in the tangential fields or with axillary radiation. This issue is being investigated in an EORTC phase III randomized trial213
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree