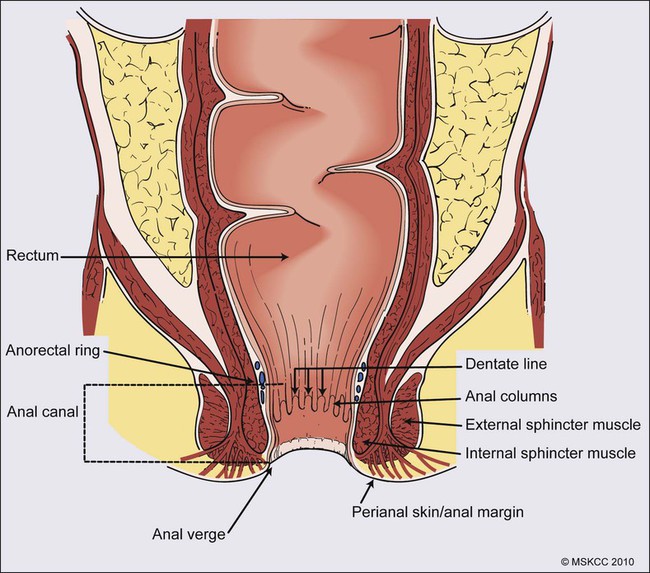
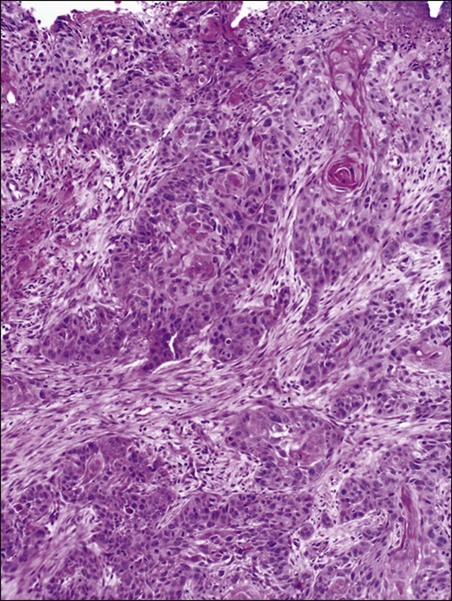
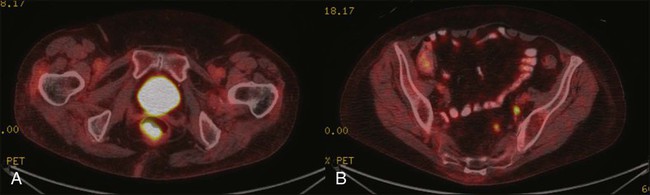
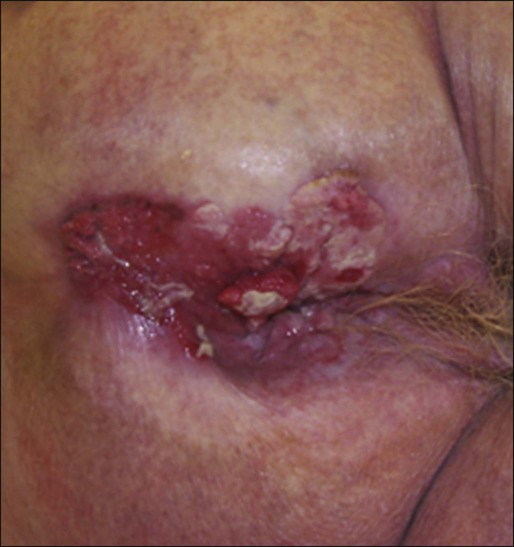
Karyn A. Goodman, Lisa A. Kachnic and Brian G. Czito • It was estimated that there were 6230 anal squamous cell carcinomas diagnosed and 780 deaths from this disease in the United States in 2012. • The age-adjusted incidence rate is 1.7 per 100,000 men and women per year with a female predominance. • Anal cancer is associated with human papilloma virus (HPV) infection, which mediates transformation of the anal squamous epithelium through a progression of precancerous lesions (anal intraepithelial neoplasia) to invasive cancer. • American Joint Commission on Cancer (AJCC) tumor–node–metastasis (TNM) staging system for anal cancer is a clinical staging system. Notably, the T stage is based on tumor size rather than depth of invasion as it is in other gastrointestinal cancers. • Physical examination, evaluating the size, location, mobility, and inguinal nodal involvement, is critical in staging patients and for a baseline comparison to determine response. • Computed tomography, sigmoidoscopy, endoanal ultrasound, and magnetic resonance imaging or positron emission tomography imaging are helpful in evaluation of the extent of the primary lesion, presence of regional nodal disease or distant metastatic disease. • Tumors of the anal canal are primarily squamous cell carcinomas. • Nonsquamous histologies are rare and include anal adenocarcinomas, melanomas, and sarcomas. • Primary therapy for anal squamous cell carcinomas consists of definitive chemoradiation with concurrent 5-flourouracil, mitomycin-C, and radiotherapy. • Surgical resection is only used as salvage for patients who have persistent or recurrent disease after chemoradiation. • Induction chemotherapy, radiation dose-intensification, and maintenance chemotherapy have not been shown to improve outcomes over standard chemoradiation for anal cancer. • The outcomes for anal squamous cell carcinoma are good, with a colostomy-free survival of 72% and overall survival of 78% at 5 years with pelvic radiotherapy and concurrent 5-fluorouracil and mitomycin-C. • More advanced tumors are associated with a higher risk of local recurrence and distant failure. Although rare, anal cancer provides a paradigm for organ preservation in the management of cancer. The treatment for anal cancer has evolved from the abdominoperineal resection (APR), which required a permanent colostomy, to sphincter-preserving nonsurgical therapy with concurrent radiation therapy and 5-fluorouracil (5-FU) and mitomycin-C chemotherapy.1,2 However, the favorable disease-free survival associated with definitive chemoradiotherapy has been tempered by a significant rate of both acute and long-term toxicities. Improvements in pelvic radiotherapy techniques to minimize the exposure of normal tissues to potentially damaging radiation doses have demonstrated promising results in reducing acute toxicities. Investigations into alternative chemotherapeutic regimens have not yielded positive results to date. However, studies evaluating targeted agents and less toxic regimens are ongoing. Further studies are warranted to assess intensification of therapy in more advanced anal cancers and reduction of treatment in early-stage disease with the goal of improving both the tolerance of chemoradiotherapy and long-term, disease-free quality of life. The anal canal is defined as the stratified squamous epithelium that extends from the anal verge to the anorectal ring, measuring approximately 3 to 4 cm in length (Fig. 79-1). The anal margin or perianal skin is the hair-bearing skin within a 5-cm radius immediately beyond the anal verge, and neoplasms involving the anal margin have been traditionally managed as skin cancers.5–5 The anal verge is, therefore, the junction of stratified squamous epithelium of the anal canal and the keratinized squamous epithelium of the perianal skin. The anorectal ring is comprised of a muscular bundle at the junction of the internal sphincter, puborectalis, and external sphincter, and the mucosa overlying the anorectal ring is a columnar epithelium. The columns of mucosa from the anorectal ring extend about 1 cm to the dentate line, the histologic transitional zone between the columnar and stratified squamous epithelium. Tumors arising near the dentate line are referred to as cloacogenic or transitional cell, and are comprised of nonkeratinizing squamous cells; however, such tumors have similar prognoses and are treated in the same fashion as squamous cell cancers of the anal canal.6 Tumors that develop from mucosa (columnar, transitional, or squamous) are true anal canal cancers, whereas tumors that arise from skin distal to the anal verge are termed anal margin or perianal skin tumors. Squamous cell carcinoma of the anus is relatively rare and comprises only 4% of all large bowel cancers.7 The National Cancer Institute’s (NCI) Surveillance of Epidemiology and End Results (SEER) Cancer Statistics Review estimated that 6230 new cases will be diagnosed in the United States and 780 deaths from the disease in the United States in 2012.7 From 2005 to 2009, the median age at diagnosis was 60 years of age.8 The age-adjusted incidence rate was 1.7 per 100,000 men and women per year, with a slight female predominance (1.9 per 100,000 women and 1.5 per 100,000 men). There has been a steady increase in incidence of anal cancer in recent years with an annual percent change of 2.2% from 1975 to 2009. Fortunately, at diagnosis, 50% of patients present with localized (stages I/II) disease and only 13% present with metastatic disease. The 5-year overall survival rate for all patients diagnosed between 2002 and 2008 was 65%, with 80% of patients with localized disease surviving 5 years.8 There have been several factors that have been implicated in the pathogenesis of anal cancer, including human papilloma virus (HPV), immunosuppression, and cigarette smoking. The most important risk factor for squamous cell anal cancer is infection with HPV, especially types 16 and 18. These high-risk HPV types act as carcinogens in the development of anogenital cancers.9,10 A recent meta-analysis suggests that HPV16 is found more frequently (75%) and HPV18 less frequently (10%) in anal carcinomas than in cervical carcinomas.11 Moreover, approximately 80% of anal cancers demonstrated more than one HPV genotype.11 As in cervical neoplasia associated with HPV, the viral proteins E6 and E7 mediate oncogenic transformation of the anal squamous epithelia. The viral E6 protein binds to the E6-associated protein (E6 AP) and ubiquinates the protein p53, which in turn leads to the proteasomal degradation of this major cellular transcription factor, leading to loss of the cell-cycle arrest and apoptotic mechanisms that allow for deletion of errors in DNA replication.12 The viral E7 protein binds to the product of the retinoblastoma (Rb) gene, and consequently accelerates the cell cycle, allowing cells to progress from the G1 into the S phase of the cell cycle.13 As anal lesions progress from condylomata to low-grade dysplasia and then high-grade anal intraepithelial neoplasia (AIN) and invasive cancer, there is an accumulation of mutant p53 expression, emphasizing its role in tumor development.14,15 A prior history of anal condylomas has been reported in as many as 50% of homosexual and 30% of heterosexual patients diagnosed with anal carcinoma.16 The E2 protein allows HPV to escape intracellular detection by facilitating attachment of the HPV DNA to the host chromatin, an effect that allows steady replication of the virus in tandem with epithelial cells.17 Therefore, through concerted actions of HPV proteins E2, E6, and E7, the anal epithelium accumulates genetic errors leading to proliferation and eventually resulting in carcinogenesis. Immunosuppression, in particular impaired cellular-mediated immunity, is another important risk factor in the development of anal cancer. The impact of suppressed cell-mediated immunity may be related to a reduced host response that would clear the HPV virus and prevent the establishment of a prolonged viral presence. Support for this finding comes from the observation that anal cancer rates are increased in both human immunodeficiency virus (HIV)–positive patients and patients that undergo renal transplantation where cell-mediated immunity is suppressed.20–20 In one series, there was an approximate 100-fold increased risk of developing anogenital cancer in renal transplant patients.19 In another series, patients receiving chronic steroid therapy for amelioration of autoimmune disorders were also found to be predisposed to HPV-associated anal lesions.21 Cigarette smoking is also associated with the development of anal cancer, much as it is with cervical cancer.22 The risk of anal cancer appears to be related to pack-year history of smoking, with more extensive histories associated with a higher risk. The mechanism of smoking-associated tumors is unclear, but smoking may act as a cocarcinogen in the context of HPV infection.23 AIN is the precursor lesion to invasive anal cancer. AIN is subdivided into low- and high-grade AIN, analogous to the classification of low- and high-grade squamous intraepithelial lesions of the cervix. Similar to the Papanicolaou (Pap) smear for cervical cancer, screening for anal cancer can be performed using anal cytology obtained by swabbing the anal canal (“anal Pap smear”). Sensitivity for detection of dysplasia appears higher (approximately 75%) in HIV-positive patients as opposed to HIV-negative patients (approximately 60%).24 In patients with abnormal cytology, anoscopy with administration of 3% acetic acid can then be performed to guide biopsies, much as is done with cervical colposcopy. Treatment of high-grade AIN includes ablation with anoscopic-directed electrocautery, topical trichloroacetic acid, topical 5-FU, or imiquimod.27–27 Topical applications yield lesion control in the range of 60% to 80%. To date, however, there are no established guidelines for anal cancer screening using anal Pap smears in high-risk groups as there are for cervical cancer screening, and cost-effective analysis showed no benefit of annual screening in HIV-positive men who have sex with men.28 There has been significant progress in the prevention of HPV-related malignancies with the introduction of the HPV vaccines. Two vaccines (Cervarix and Gardasil) are now approved by the U.S. Food and Drug Administration and have been shown to protect against cervical cancer in women.29,30 The quadrivalent HPV vaccine Gardasil has a high efficacy for prevention of HPV 6-, 11-, 16-, and 18-related genital warts and has been shown to protect against cancers of the anus, vagina, and vulva.31 The quadrivalent HPV vaccine was also studied in men who have sex with men and was found to reduce the rates of AIN.18 Both vaccines have a favorable safety profile. HPV vaccination is now recommended by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control for preteen girls and boys at age 11 or 12 years and girls 13 to 26 years who have not been previously vaccinated. A variety of malignancies can arise within the anal canal. Simplistically, these can be divided into squamous and nonsquamous histologies. Squamous cell tumors are the most common presentation. Historical histologic descriptors such as basaloid, cloacogenic, and junctional (tumors arising in the transitional mucosa of the proximal canal described above) are not as relevant for practical management and are not included in the current World Health Organization classification system for anal cancer. Tumors arising in this area are considered squamous cell tumors (generally nonkeratinizing) and are managed accordingly. Tumors arising below the dentate line are often keratinizing squamous cell carcinomas (Fig. 79-2). Nonsquamous cell tumors including adenocarcinomas, melanomas, lymphomas, neuroendocrine tumors, and sarcomas have been described, but are less common. A suspected anal adenocarcinoma may actually represent extension from a distal rectal adenocarcinoma in some scenarios. Adenocarcinoma arising in the anal canal has a different clinical biology than squamous cell carcinoma and is usually managed like rectal cancer. Ultimately, tumor location within the anal canal is not as important as histologic subtype. The regional nodes of the anal canal are considered to be inguinal (superficial and deep femoral), internal iliac, external iliac, and perirectal (mesorectal). All other nodal groups represent sites of distant disease. The incidence of involvement of inguinal nodes is directly proportional to the size and extent of the primary tumor. Of all patients presenting with palpable inguinal lymph nodes, only approximately 50% will contain cancer; therefore, fine-needle aspiration is recommended in suspected cases.32 Anal cancer is primarily a locoregional disease, with only approximately 10% of patients diagnosed with distant metastases at presentation. The liver and lungs are the most frequent site of distant spread. After curative treatment, the risk of distant disease varies and depends on the initial tumor size and nodal stage.33 The most common presenting symptom in patients with anal cancer is rectal bleeding, occurring in approximately one-half of patients.34,35 Because bleeding is frequently minor, often in conjunction with a mass palpated at or above the anal sphincter, an initial errant diagnosis of hemorrhoids is common. Additional presenting symptoms include pain from local invasion or a sensation of anorectal fullness, occurring in approximately 30% of patients,20 pruritus, changes in bowel habits, and tenesmus. Frank incontinence is less common but is suggestive of more advanced disease and sphincter destruction. In a minority of presentations, disease is discovered on routine physical examination in an otherwise asymptomatic patient. Physical examination will often reveal a relatively indurated mass with potential bleeding and ulceration (Fig. 79-3). This may be visible on direct inspection with disease at or distal to the anal verge. On digital examination, tumor location, size, involvement of adjacent organs, and extent should be fully appreciated, including relationship of the mass to the dentate line by anoscopy/endoscopy. Histologic confirmation of malignancy by tissue biopsy is required prior to initiation of tumor-directed therapy, and any suspicious mass should undergo incisional biopsy. If there is clinical suspicion of malignancy prior to biopsy, full local excision should generally be reserved for very small, superficial lesions. In female patients, vaginal examination should be performed to rule out posterior vaginal invasion and fistula, and evaluation of the cervix should be performed to rule out gynecologic malignancy. Given patterns of spread, thorough examination of lymph node areas, including inguinofemoral regions, should be performed. Any suspicious lymphadenopathy should undergo biopsy, generally through fine-needle aspiration, to further facilitate diagnosis and tumor staging. Table 79-1 reviews the standard evaluation for patients with anal cancer. Table 79-1 Recommended Diagnostic Evaluation in Patients with Anal Cancer Once diagnosis is established, patients should undergo imaging for staging, including contrasted chest, abdominal and pelvic computed tomography (CT), often in conjunction with positron emission tomography (PET) scan. HIV testing should be performed in patients with established risk factors. Staging, as with most tumors, involves determining local extent of the primary disease, whether or not nodal metastases are present, and whether or not there is evidence for distant spread of disease (outside of the pelvis). PET and PET-CT are now also routinely integrated into the staging algorithm for patients (Fig. 79-4).38–38 In one series, PET-CT appeared to have a higher sensitivity than conventional imaging (CT and/or magnetic resonance imaging [MRI]) for detecting regional lymph node metastases (89% vs. 62%), although for practical reasons not all nodes could be biopsied for a true measure of sensitivity and specificity. PET was found to change planned radiation therapy fields in 13% of patients and thus was worthy of inclusion in the staging process.38 In other reports, PET-CT upstaged 17% to 38% and downstaged 19% to 25% of patients; similarly, there was also change in management in 13% to 29% of patients.39,40 Of note, HIV-positive patients may have falsely positive fluorodeoxyglucose–avid lymph nodes. Biopsy may be necessary in these situations to determine whether or not there is true nodal metastatic disease versus a benign inflammatory process. Table 79-2 outlines the American Joint Commission on Cancer (AJCC) staging system. Table 79-2 AJCC Staging System for Anal Cancer, Seventh Edition (2010) Note: cTNM is the clinical classification, pTNM is the pathological classification. *Direct invasion of the rectal wall, perirectal skin, subcutaneous tissue, or the sphincter muscle(s) is not classified as T4. Used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer, New York, Inc. Until the mid-1970s, surgery was the gold standard for the treatment of anal canal cancer. The standard surgical technique was an APR, requiring a permanent colostomy as well as removal of the rectum, ischiorectal fat, levator sling, perirectal and superior hemorrhoidal nodes, and a wide area of perianal skin. Long-term sexual and urinary dysfunction are potential consequences of an APR. The 5-year overall survival (OS) rate for APR for all patients with anal cancer was approximately 50%.41,42 For superficial lesions without lymph node involvement, the Mayo Clinic reported 5-year OS rates of 90% with radical surgery; however, with muscle invasive, node-positive disease, outcomes were poor, with a 5-year OS of 32%.43 A subsequent review from the Mayo Clinic of 118 anal canal cancers treated with an APR showed an OS rate of 70% and an overall recurrence rate of 40%. Over 80% of those with known recurrence sites had either exclusively local recurrence or a local recurrence component.44 Frost and colleagues45 reported a 5-year OS of 62%, with a 45% rate of failure in the pelvic and inguinal lymph nodes. Given the poor outcome with radical surgery alone, Nigro evaluated the use of preoperative chemoradiation therapy for anal canal squamous cell cancer. The first report of three patients who received radiation therapy to 30 Gy in 15 fractions, with concurrent 5-FU (25 mg/kg with continuous infusion [CI]) and mitomycin (0.5 mg/kg bolus), was published in 1974.46 Two patients underwent subsequent APR, and one patient refused surgery. At the time of APR, both patients showed pathological complete response, and the patient who refused surgery had no evidence of disease clinically at 14 months.46 Nigro subsequently reported results for a total of 28 patients with anal squamous cell cancer treated in the same manner with preoperative radiation therapy and chemotherapy. Surgery was performed 4 to 6 weeks following the last day of radiation treatment. Twelve patients underwent APR, of whom seven had no residual tumor in the surgical specimen, while one patient had microscopic tumor only. An additional 14 patients had a complete clinical response, and underwent local excision that confirmed pathological complete response. Two other patients were clinically free of tumor without biopsy confirmation.47,48 In a subsequent series using definitive chemoradiation as primary therapy, 38 of 45 patients were cured of disease, with a 5-year OS of 67% and colostomy-free survival of 59%.49 These data suggested that definitive chemoradiation could be used to treat anal canal cancer with equivalent or better tumor control and significantly reduced mortality compared to APR. APR is now rarely used as initial therapy; however, it is still an option for salvage after persistence or recurrence of disease, or for management of complications after combined-modality therapy.50 For early-stage, small (<2 cm), well-differentiated anal canal cancers without other adverse histologic features that are not invading the underlying sphincter and have no clinical lymph node involvement, local excision is considered an option. For these highly selected patients, OS at 5 years has been reported to be over 80%.4,42 However, local excision should only be considered for lesions of the anal margin in which the sphincter can be spared. Several large randomized multiinstitutional studies, detailed below, have validated definitive chemoradiation with concurrent 5-FU and mitomycin as the primary treatment modality for anal canal cancer.51–56 Table 79-3 summarizes the key findings. Combined-modality therapy has been compared to radiation alone in several trials, which have all demonstrated the superiority of chemoradiation for sphincter preservation. In general, primary radiotherapy alone has lower treatment-related toxicity than chemoradiation, but is associated with a higher local failure rate in tumors greater than 2 cm. Table 79-3 Summary of Major Randomized Phase III Clinical Trials of Chemoradiotherapy for Anal Cancer
Cancer of the Anal Canal
Introduction
Anatomy
Epidemiology
Etiology and Biological Characteristics
Prevention and Early Detection
Pathology and Pathways of Spread
Clinical Manifestations/Patient Evaluation/Staging

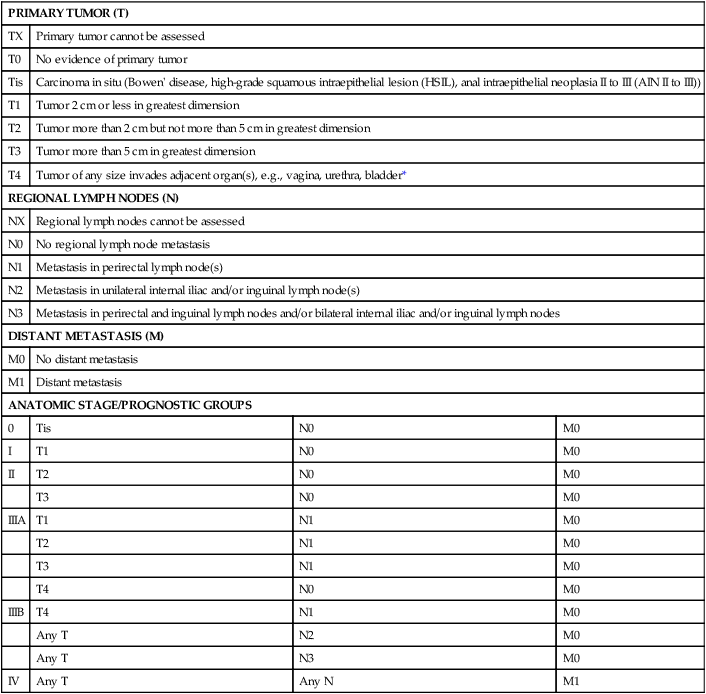
PRIMARY TUMOR (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ (Bowen’ disease, high-grade squamous intraepithelial lesion (HSIL), anal intraepithelial neoplasia II to III (AIN II to III))
T1
Tumor 2 cm or less in greatest dimension
T2
Tumor more than 2 cm but not more than 5 cm in greatest dimension
T3
Tumor more than 5 cm in greatest dimension
T4
Tumor of any size invades adjacent organ(s), e.g., vagina, urethra, bladder*
REGIONAL LYMPH NODES (N)
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Metastasis in perirectal lymph node(s)
N2
Metastasis in unilateral internal iliac and/or inguinal lymph node(s)
N3
Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes
DISTANT METASTASIS (M)
M0
No distant metastasis
M1
Distant metastasis
ANATOMIC STAGE/PROGNOSTIC GROUPS
0
Tis
N0
M0
I
T1
N0
M0
II
T2
N0
M0
T3
N0
M0
IIIA
T1
N1
M0
T2
N1
M0
T3
N1
M0
T4
N0
M0
IIIB
T4
N1
M0
Any T
N2
M0
Any T
N3
M0
IV
Any T
Any N
M1
Primary Therapy
Squamous Cell Carcinoma
Surgery
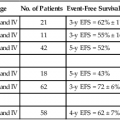
Prospective Trials Evaluating Combined-Modality Therapy
Study (Reference)
Treatment Arms*
Local-Regional Failure†
Relapse-Free Survival†
Colostomy-Free Survival†
Overall Survival
Acute Toxicity
UKCCCR/ACT I51,51a
(1) Radiation
(1) 59.1%
(1) 17.7%
(1) 20.1%
(1) 27.5%
(1) “Severe” skin toxicity = 39%; “severe” GI toxicity = 5%
(2) Radiation + 5-FU + MMC
(2) 33.8% (at 12 years)
(2) 29.7% (at 12 years)
(2) 29.6% (at 12 years)
(2) 33.1% (at 12 years)
p=NS
(2) “Severe” skin toxicity = 50%; “severe” GI toxicity = 14%
EORTC53
(1) Radiation
(2) Radiation + 5-FU + MMC
18% improvement in arm 2 at 5 years
N/A
32% improvement in arm 2 (colostomy free)
(1) Grades 3–4 diarrhea = 8%; grades 3–4 dermatologic = 50%
(2) Grades 3–4 diarrhea = 20%; grades 3–4 dermatologic = 57%
RTOG/ECOG54
(1) Radiation + 5-FU
(1) 34%
(1) 51%
(1) 59%
(1) 67%
(1) Grades 4–5 heme = 3%; grades 4–5 non-heme = 4%
(2) Radiation + 5-FU + MMC
(2) 16%
(2) 73% (disease-free survival at 4 years)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access