Key points
- 1.
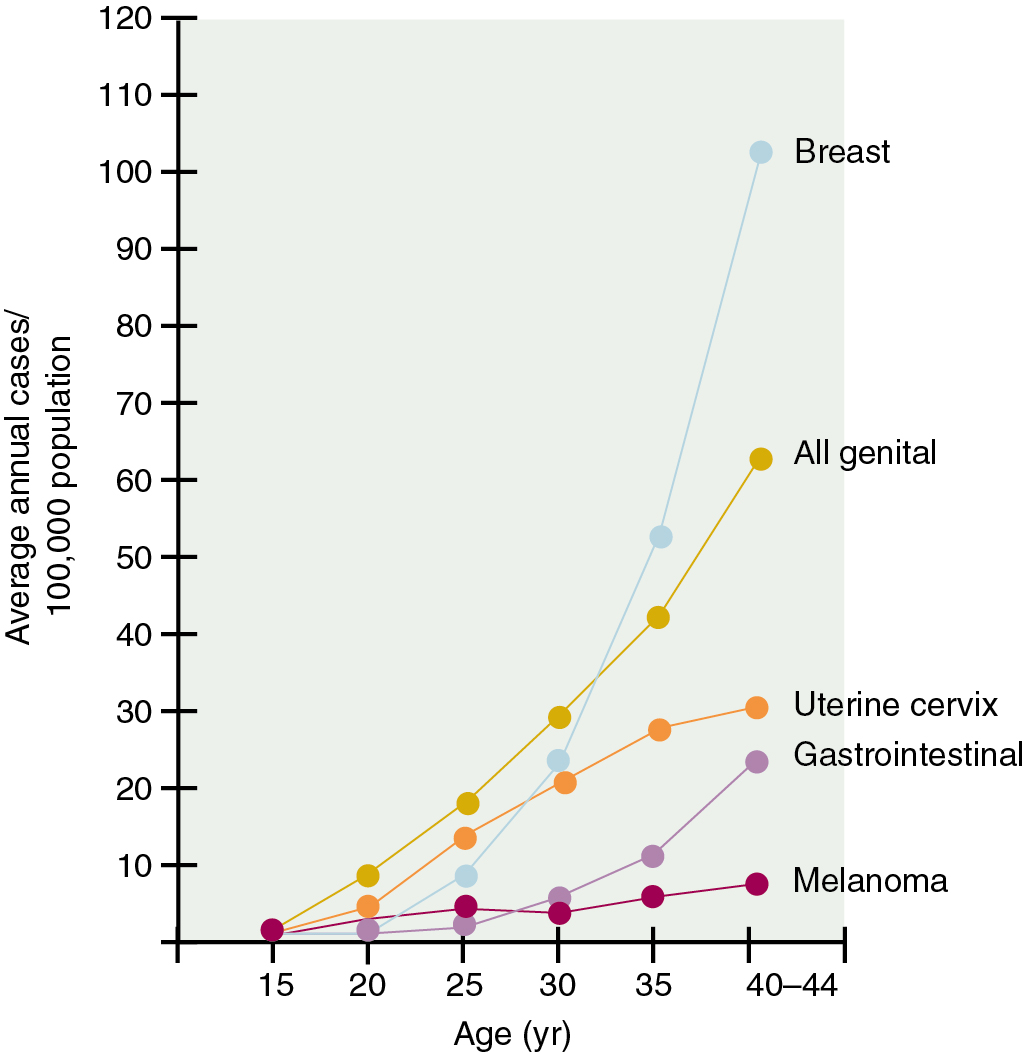
Data from the Surveillance, Epidemiology, and End Results database indicate that cancers during the reproductive ages, in order of decreasing frequency are breast, melanoma, thyroid, cervix, and lymphoma.
- 2.
The most critical period for cancer treatment during pregnancy extends from the 3rd to 8th weeks of development (5th through 10th weeks of gestational age), when susceptibility to teratogenic agents is maximal. In the human fetus, the period of organogenesis usually ends by the 13th week of gestation.
- 3.
Preterm babies have limited ability to metabolize drugs because of immaturity of the hepatic and renal systems. Neonates exposed to chemotherapy 3 weeks before delivery should be assessed for transient bone marrow suppression, and long-term neurologic and developmental follow-up is recommended.
- 4.
As more women opt for conservative management of invasive cancers in an effort to preserve fertility, we may anticipate a larger number of referrals than what was seen previously for patients with persistent or even recurrent malignancy during pregnancy.
- 5.
Many recommend that any child born to a mother with active or suspected malignancy should initially have a thorough physical examination with a complete blood count, comprehensive metabolic panel, liver function tests, coagulation battery, serum lactate dehydrogenase level, uric acid levels, and a urinalysis. In addition, the placenta should be macroscopically and microscopically examined for tumor involvement. It has been our practice to also obtain imaging studies, including magnetic resonance imaging of the brain and computed tomography scans of the chest, abdomen, and pelvis, when maternal breast cancer, hematopoietic malignancy, or melanoma is at issue or in the setting of confirmed placental metastases.
Background and epidemiology of cancer
Cancer in pregnancy poses significant challenges to both the clinician and the pregnant woman. This is undoubtedly the result of the trend to defer childbearing into the fourth decade of life, when the incidence of some of the more common malignant neoplasms begins to rise ( Fig. 12.1 ). The tragedy of the presence of a malignant neoplasm discovered during pregnancy raises many issues ( Table 12.1 ). Fortunately, the peak incidence years for most malignant diseases do not overlap the peak reproductive years ( Table 12.2 ). Thus, as in any unusual situation that physicians rarely encounter, clear therapeutic decisions are not readily at hand. However, a significant number of well-studied reviews can provide some guidance in this dilemma. The largest series ever reported was that of Barber and Brunschwig in 1968, which consisted of 700 cases of cancer in pregnancy. The most common malignant neoplasms in that series were breast tumors and leukemias–lymphomas as a category, melanomas, gynecologic cancer, and bone tumors, in that order. Other authors suggest that gynecologic malignant neoplasms are second only to breast carcinoma and remind us that cancer of the colon and thyroid are also seen in pregnancy ( Table 12.3 ).
| Oncologic Issues | Obstetric Issues | Ethical, Religious, Medicolegal, and Socioeconomic Issues |
|---|---|---|
| Timing of surgery Type of therapy Maternal effects of therapy Maternal surveillance Fertility-sparing therapy | Fetal effects of therapy Antepartum fetal surveillance Corticosteroid administration Amniocentesis Timing of delivery Route of delivery Neonatal effects of therapy Long-term effects of therapy | Pregnancy termination Independent advocate for the fetus Fetal viability Maternal risk Healthcare costs Principle of beneficence Right to autonomy Mother’s overall prognosis a |
a It is important to take into account the estimated length of time the mother will realistically live to spend with the baby.
| Birth–39 Years | 40–59 Years | |
|---|---|---|
| All sites | 2.07 (1 in 48) | 8.97 (1 in 11) |
| Breast | 0.48 (1 in 208) | 3.79 (1 in 26) |
| Melanoma | 0.27 (1 in 370) | 0.58 (1 in 189) |
| Uterine cervix | 0.15 (1 in 651) | 0.27 (1 in 368) |
| Leukemia | 0.12 (1 in 835) | 0.14 (1 in 693) |
| Non-Hodgkin lymphoma | 0.08 (1 in 1191) | 0.32 (1 in 316) |
| Colon and rectum | 0.07 (1 in 1343) | 0.72 (1 in 138) |
| Uterine corpus | 0.07 (1 in 1499) | 0.72 (1 in 140) |
| Lung and bronchus | 0.03 (1 in 2997) | 0.81 (1 in 124) |
| Urinary bladder | 0.01 (1 in 10,185) | 0.12 (1 in 810) |
| Patients ( n ) | Patients (%) | |
|---|---|---|
| Breast cancer | 99 | 46 |
| Hematologic malignancies | 40 | 18 |
| Dermatologic malignancies | 21 | 10 |
| Cervical cancer | 17 | 8 |
| Brain tumor | 8 | 4 |
| Ovarian cancer | 8 | 4 |
| Colorectal cancer | 5 | 2 |
| Other (e.g., sarcoma, lung, liver, kidney, GI) | 17 | 8 |
The incidence of cancer in pregnancy is unclear but is estimated to be 1 in 1000. From historical case series collected at a variety of referral institutions, many commentators have concluded that cervical cancer is the most frequent malignancy to complicate pregnancy. This finding is likely to be inaccurate because the incidence of cervical cancer in the United States and in most developed nations is steadily declining. In a 1984 population-based study, Haas reviewed the National Cancer Registry of the German Democratic Republic for the years between 1970 and 1979, and from a total of 31,353 cancer cases and 2,103,112 live births among women between the ages of 15 and 44 years, 355 pregnant women were diagnosed with a malignancy. Dinh and Warshal emphasized that in the Haas study, the incidence of cancer in pregnancy per 1000 live births rose from 0.02 for women aged 15 to 19 years to 2.3 for women aged 40 to 44 years. In order of decreasing frequency, cancer of the cervix, breast cancer, ovarian cancer, lymphoma, melanoma, brain cancer, and leukemia were found to complicate pregnancy.
In a recent study using data from the Swedish Multi-Generation Register and the National Cancer Registry from 1963 to 2007, Andersson et al. noted that the three most common malignancies during pregnancy were melanoma ( n = 232), breast cancer ( n = 139), and cervical cancer ( n = 139). Interestingly, with a slightly different rank order, these cancers are also the most common to occur in women of childbearing age. Overall the investigators believed that the number of observed cases during pregnancy was lower than expected for all cancers, the reasons of which were unclear. In this study, it was noteworthy that a rebound in the number of observed cases during the postpartum period was restricted to melanoma, nervous system malignancies, and breast and thyroid cancer.
Data from the Surveillance, Epidemiology, and End Results (SEER) program in the United States from 1992 to 1996 estimate that among women aged 15 to 44 years, in order of decreasing frequency, cancer of the breast, melanoma, thyroid cancer, cervical cancer, lymphoma, and ovarian cancer are coincident with pregnancy. The Centers for Disease Control and Prevention have highlighted pregnancy trends in the United States over the preceding 25 years. Although the birth rate for women younger than 30 years of age rose slowly until the early 1990s, it has steadily declined since then. In contrast, the birth rate for women older than 30 years of age has risen steadily over the past 2 decades by an average of 67%. Because of the changing attitudes regarding the role of women as part of the workforce, the delay in childbearing observed in this country will be associated with at least three considerations that are germane to the subject of this chapter:
- 1.
Because many malignancies manifest with advancing age, it is reasonable to expect an increase in the occurrence of some specific types of cancers during pregnancy.
- 2.
Theoretic concerns regarding possible effects of pregnancy-derived hormones among cancer survivors contemplating pregnancy will need to be addressed.
- 3.
With the popularization of investigational fertility-sparing medical and surgical therapy for nulliparous patients with seemingly early lesions who strongly desire to retain childbearing capacity, there exists an increased potential for the oncologist to encounter recurrent disease irrespective of whether pregnancy occurs.
The enormous physiologic changes of pregnancy suggest many possible influences on the malignant state. First, it has been assumed by many that malignant neoplasms arising in tissues and organs influenced by the endocrine system are possibly subject to exacerbation with pregnancy, and this has often been erroneously extrapolated to a recommendation for “therapeutic” abortion. Second, the anatomic and physiologic changes of pregnancy may obscure the subtle changes of an early neoplasm. Third, the increased vascularity and lymphatic drainage may contribute to early dissemination of the malignant process. Although all these hypotheses are interesting, the validity of each is variable, even within the same organ.
Several additional points must be emphasized when caring for a pregnant patient with symptoms suggestive of cancer or in whom the diagnosis has been established. Although pregnancy is usually characterized by extensive medical observation, a delay in diagnosis can occur if attention is not paid to the subtle presentation of malignancies. Thus, although pregnancy has not been shown to increase the virulence of any tumor type, many pregnancy-associated cancers portend a poor prognosis for the woman. Even though many essayists have claimed that the conduct of the pregnancy is not affected by the cohabitation of malignancy, the oncologist must recognize that some tumor types have been shown to metastasize to the placenta and even to the fetus. In all cases of pregnancy complicated by malignancy, it is advisable to have a multidisciplinary team of specialists involved in the care of the patient.
When considering therapy in pregnancy, surgery is rarely contraindicated, with the optimal time being in the second trimester. Chemotherapy for the most part should have restricted use during the first trimester but can generally be safely administered thereafter. Certain diagnostic imaging procedures can be safely performed during pregnancy, but in most cases, radiation therapy should be postponed until after delivery. Aggressive nutritional support is a mandatory requirement for pregnant women with cancer. In the majority of cases, with a proper coordination of effort, the pregnancy need not be terminated to begin treatment.
Report from the european society of gynecological oncology cancer in pregnancy task force
Amant et al. recently reported updated guidelines from the Second International Consensus Meeting arranged to disseminate experts’ knowledge and form consensus guidelines for medical and oncologic intervention to address maternal malignancy diagnosed during pregnancy and the postpartum period. Protocols for management of pregnancy-associated cervical cancer, ovarian cancer, and vulvar cancer were reviewed. The guidelines developed advocate following standard treatment protocol as for nonpregnant patients to maximize maternal outcome but with the caveat that iatrogenic prematurity should be avoided. The experts committee stressed effective psychologic support throughout the pregnancy and endorsed diagnostic procedures including open or laparoscopic surgical staging examinations, as well as imaging being preferably performed using nonionizing radiation modalities such as magnetic resonance imaging (MRI) and ultrasonography. Importantly, most chemotherapy regimens were thought to be tolerable and safe from 14 weeks’ gestational age onward. Although term delivery should be the goal, apart from cervical and vulvar cancer (with concomitant vulvar scarring), the mode of delivery should be based in most cases on obstetric indications.
In 2014, the European Society of Gynaecological Oncology (ESGO) Cancer in Pregnancy Taskforce was transformed to the International Network of Cancer, Infertility, and Pregnancy (INCIP), which now includes contributions from 62 centers from 25 different countries. The aim of the INCIP is to report on obstetric and oncologic outcomes of patients with cancer in pregnancy. As of August 2019, INCIP had enrolled 2059 patients with a cancer diagnosis or undergoing cancer treatment during pregnancy, 395 patients who underwent fertility preservation, and 199 patients who were diagnosed with cancer within 2 years of delivery. The majority of the enrolled patients come from Belgium, the Netherlands, Italy, and the United States, with breast (40%), lymphoma (12%), and cervical (10%) being the most common cancer types represented.
The more common solid tumors
Cervical cancer
Cervical cancer complicates approximately 1 in 1200 pregnancies. As a consequence of widespread cytologic screening, the dramatic decrease in invasive cervical cancer observed in recent years has been paralleled by a rise in cervical intraepithelial neoplasia (CIN), especially in younger women. Because the peak incidence for both CIN and childbearing occurs during the third decade of life, abnormal Papanicolaou (Pap) tests are common among gravid women, occurring at a rate of 0.5% to 5%. The diagnosis of cervical dysplasia in pregnancy may occur in up to 5% of some populations. For these reasons, screening for cervical neoplasia is an essential component of prenatal care. All pregnant women should have a Pap test submitted for cytology. The ectocervix and endocervical canal should be sampled adequately. Patients noted to have a visible lesion should undergo cervical biopsy immediately because cervical cytology taken directly from tumors often contain only inflammatory cells. A recent review from the Magee Gynecologic Cancer Program in Pittsburgh noted that in some populations, up to 20% of pregnant women have an abnormal Pap result during pregnancy. Nearly 3% of newly diagnosed cervical cancer cases occur in pregnant women, probably because it is the one cancer that is screened for as part of routine prenatal care.
In concordance with the known risk factors for invasive cervical cancer, pregnant women who develop CIN tend to marry at an earlier age, have a higher parity, and are diagnosed at an earlier age than nonpregnant women with CIN. Hacker and colleagues compiled data from nine reports and noted that the average age of patients with carcinoma in situ (CIS) during pregnancy was 29.9 years, and the average parity was 4.0. Among nonpregnant women, the average age of CIS is 35 years. The investigators noted that the median age of patients diagnosed with invasive carcinoma of the cervix during pregnancy is 33.8 years (range, 17 to 47 years), and the average parity is 4.5. The average parity among pregnant women with cervical cancer was 5.4 in a study reported by Creasman and colleagues; in this group, increasing parity was not associated with a more advanced lesion, nor did it have an impact on prognosis.
Human papillomavirus in pregnancy
Although human papillomavirus (HPV) is strongly associated with cervical dysplasia and carcinoma in both nonpregnant and pregnant women, a significant relationship between pregnancy and HPV prevalence has not been established. Eversion of the endocervical epithelium results in exposure to the acidity of the vaginal environment, producing a high degree of squamous metaplasia. This metaplasia is important because HPV requires active cellular machinery to reproduce and transform cells. Schneider and colleagues examined the negative cervical smears of 92 pregnant and 96 nonpregnant, age-matched control participants for the presence of HPV DNA by Southern blot hybridization. The investigators demonstrated both an increased prevalence of HPV (preferentially the oncolytic HPV subtype 16) and a higher replication rate of viral DNA during pregnancy. Using the ViraPap/ViraType dot blot DNA hybridization procedure, Smith and coworkers detected an increase in HPV prevalence with advancing gestational age, suggesting that as estrogen levels increase, pregnant women may be more vulnerable to HPV infection. Using similar hybridization methods, however, Kemp and colleagues and Chang-Claude and colleagues were unable to demonstrate a higher prevalence of HPV infection during pregnancy.
Castellsague and colleagues performed a prospective study in Barcelona to quantitate the mother-to-child transmission of HPV subtypes. This study included 66 HPV-positive and 77 HPV-negative pregnant women and their offspring. To estimate HPV prevalence and genotypic distribution in pregnancy, the investigators also carried out a related screening survey of cervical HPV DNA detection among 828 pregnant women. Exfoliated cells from the mouth and external genitalia of the infants were collected at birth and at several intervals up to 2 years of age. At 418 infant visits and a mean follow-up time of 14 months, 19.7% of infants born to HPV-positive mothers and 16.9% of those born to HPV-negative mothers tested HPV positive at some point during the infants’ follow-up. The most frequently detected genotype both in infants and in mothers was HPV-16. Of note, there was a strong and statistically significant association between mother’s and child’s HPV status at the 6-week postpartum visit in that children of mothers who were HPV positive at the postpartum visit were five times more likely to test HPV positive than children of corresponding HPV-negative mothers ( P = .02). The authors concluded that the risk of vertical transmission of HPV genotypes is relatively low and that vertical transmission may not be the sole source of HPV infections in infants. There exists the potential for horizontal mother-to-child transmission.
Evaluation of cervical cytology in pregnancy
The cytopathologist frequently encounters atypical cells when reviewing the cervical cytology from a pregnant patient. Cells within the endocervical canal that undergo the Arias–Stella reaction may contain a vacuolated clear or oxyphilic cytoplasm, intraglandular tufts, hobnail patterns, delicate filiform papillae, intranuclear pseudoinclusions, cribriform intraglandular growth, and even occasional mitotic figures. Distinguishing features of dysplastic and frankly malignant cells would include an infiltrative pattern, spectrum of cytologic atypia, a high nuclear-to-cytoplasmic ratio, and increased mitotic activity. Other atypical cells exfoliated by the endocervix in pregnant women include small decidualized cells with sharp cytoplasmic borders and hypochromatic nuclei, but unlike dysplastic cells, decidualized cells contain regular chromatin and distinct nuclei. Finally, large, multinucleated trophoblastic cells may be discharged from the uterus. At this time, it is not clear if liquid-based cytology can decrease the false-positive rate. Nevertheless, careful inspection of cervical cytology maintains its reliability as a screening test for dysplasia among pregnant patients.
The performance of colposcopy in pregnancy
Colposcopy is facilitated by the pregnancy-induced eversion of the normal cervical ectropion. However, pregnancy results in dramatic alterations in the colposcopic appearance of the cervix, the most significant changes resulting from the elevated levels of circulating estrogen, which produces a significant increase in cervical volume through hypertrophy of the fibromuscular stroma. The increased vascularity produces a bluish hue, which is then exaggerated with application of acetic acid to the metaplastic epithelium in pregnancy. Toward the end of the first trimester, eversion and metaplasia produce areas of fusion of columnar villa and distinct islands or fingers of immature metaplastic epithelium. Fine punctation and even mosaicism may accompany metaplasia, which in and of itself produces an acetowhite effect. Tenacious endocervical mucus develops, which further hinders colposcopic examination. Finally, stromal edema, enlargement of glandular structures, acute inflammatory responses, and stromal decidualization may occur in the second and third trimesters, which, although physiologic, may appear suspicious to the inexperienced colposcopist. For these reasons, colposcopy in pregnancy is difficult and should be reserved for an experienced gynecologist.
The aim of colposcopy in pregnancy is to exclude cancer, and only one directed biopsy of the site compatible with the most advanced area of dysplastic change should be performed to establish the histologic level of disease. Because of false-negative results ranging from 8% to 40%, random or nondirected biopsies should be avoided. Great care must be exercised because the increased vascularity may lead to precipitous, heavy bleeding. A Tischler or baby Tischler biopsy forceps should be used followed by immediate placement of a cotton-tipped applicator above the cervical epithelium. If bleeding occurs, it may be controlled with three silver nitrate sticks or with dehydrated Monsel’s solution. An endocervical curettage, however, is best avoided during pregnancy.
Yoonessi and colleagues conducted a retrospective analysis of suspected CIN associated with pregnancy and concluded that colposcopic examination with or without directed biopsy eliminated the need for cervical conization in 104 of 107 patients. In their classic paper, Hacker and colleagues noted that serious morbidity, such as hemorrhage, preterm labor, miscarriage, or infection, only infrequently occurs when directed biopsies are performed. For 1064 reported colposcopic examinations during pregnancy, the diagnostic accuracy was 99.5%, and the complication rate was 0.6%. No case of frankly invasive carcinoma was missed, and the two cases of microinvasion missed on colposcopic biopsy both had a colposcopic pattern suggestive of microinvasion, which was confirmed by subsequent conization. Thus, in experienced hands, colposcopy reduces the need for cone biopsy in pregnancy, with a false-negative rate of less than 0.5%.
Recently, Wetta and colleagues presented the University of Alabama experience on 625 pregnant women with CIN. The most common referral cytology was low-grade squamous intraepithelial lesions (LSIL; 41%) followed by atypical squamous cells of undetermined significance (ASC-US; 34.1%) and high-grade squamous intraepithelial lesions (HSILs; 13.6%). Of the 269 patients with ASC-US and LSIL cytology, 20 of 78 patients who underwent cervical biopsy were diagnosed with CIN II or III. Of the 128 patients with HSIL, 31 of 60 patients who underwent cervical biopsy were diagnosed with CIN II or III. Repeat colposcopy in the third trimester was performed on 47 patients, and only 3 of 13 patients who had a repeat biopsy had CIN II or III. The authors concluded that pregnant patients with ASC-US or Low grade squamous intraepithelial lesion (LSIL) cytology rarely have colposcopically suspected CIN II or III at their initial colposcopy that warrants a cervical biopsy. The investigators consider it reasonable to defer the initial colposcopy in these cases until at least 6 weeks postpartum.
Onuma and colleagues have evaluated the diagnosis of ASC-H (atypical squamous cells with possible HSIL) in 60 patients. Among 30 who had histologic follow-up, 3 women (10%) had HSIL, and 13 (43%) had LSIL. Among 32 women who had cytologic follow-up, 2 (6%) had HSIL, 3 (9%) had LSIL, one (3%) had ASC-H, and 3 (9%) had ASC-US. High-risk HPV DNA was detected in 24 of 43 patients (56%). The authors suggest that ASC-H in pregnant women has a lower predictive value for an underlying HSIL compared with the general population. Although a positive high-risk HPV DNA test result was not a good indicator for underlying SIL, a negative result appeared useful for ruling out an underlying HSIL. The authors advocated for a more conservative follow-up for pregnant women with ASC-H and support using high-risk HPV DNA testing as an adjunctive test.
The natural history of cervical intraepithelial neoplasia in pregnancy
It appears that in an immunocompetent host evaluated colposcopically and pathologically by experienced eyes, CIN rarely, if ever, progresses to microinvasive disease during pregnancy. In fact, there appears to be a subset of patients who will experience disease regression after delivery of the neonate. Postpartum regression rates for abnormal cervical cytology consistent with dysplasia (combining both LSIL and HSIL) have ranged from 25% to 77%. This wide range is hard to explain, with some authors postulating that regression occurs in at least one-third of patients as a consequence of resolution of pregnancy-induced changes in the maternal immunologic system. An Italian study published in 2008 detailed the natural history of CIN in 78 pregnant women. Among those with CIN II or III ( n = 36; 46.2%), no invasion was suspected during pregnancy, and at the postpartum evaluation, no invasive or microinvasive cancer was diagnosed. Of note, there were 19 (52.7%) cases of persistent CIN II or III and 42 (53.8%) regressions. The authors noted that CIN I has a significantly higher tendency to spontaneous regression compared with nonpregnant women with CIN I. High-risk HPV testing may improve the follow-up of patients with SIL in pregnancy and postpartum to assist in the diagnosis of persistent infections.
Some authors have advanced the theory that vaginal birth trauma may result in the complete debridement of dysplastic tissues. This phenomenon was observed by Ahdoot and colleagues in a prospective collection of abnormal cytology during pregnancy and in the postpartum period. The investigators observed a 60% regression rate among women with HSILs who delivered vaginally versus 0% in those with HSILs who delivered by cesarean section ( P < .0002). A study by Siristatidis and colleagues demonstrated a 66.6% regression rate among women with HSILs who delivered vaginally versus 12.5% of those with HSILs who delivered by cesarean section ( P < .002). In direct contradistinction, the cytologic study by Murta and colleagues (LSILs in pregnancy) and the pregnancy-related histologic investigations by Murta and colleagues (CIN CIN II or III), Yost and colleagues (CIN II or III), and Coppola and colleagues (CIS) failed to show any statistically significant difference in postpartum regression rates for patients who delivered vaginally versus those who labored and went on to deliver by cesarean section versus those who underwent elective cesarean delivery.
A recent report by Ueda and colleagues describes the experience with CIN in pregnancy at Osaka University Hospital. The investigators observed regression of CIN in 34 (76%) of 45 cases of vaginal delivery and in 6 (50%) of 12 cases of cesarean delivery, indicating that the outcome of an initially diagnosed CIN and the delivery routes appear not to be significantly related. However, a different result was obtained when only patients whose CIN lesions persisted until the delivery were analyzed. Among the 35 such cases in the vaginal delivery group, 24 cases (69%) regressed after the delivery; in 8 such cases from the cesarean delivery group, only 2 cases (25%) regressed after delivery. There was also significantly more frequent postpartum regression of biopsy-proven CIN lesions after vaginal delivery compared with cesarean section ( P = .042; odds ratio [OR] 6.55; 95% confidence interval [CI], 1.13 to 37.8).
Conization and related procedures in pregnancy
The performance of a cone biopsy during pregnancy is a formidable undertaking, and one must weigh the risks of the procedure against the anticipated yield of microinvasive carcinoma (MIC), which would remain otherwise undetected. Maternal risk appears to be restricted to either immediate or delayed hemorrhage, occurring in up to 14% of cases and exceeding 400 mL when the procedure is performed during the third trimester. Averette and colleagues reported the largest series of cold knife cervical conization biopsies in pregnancy and noted that 9.4% of the study group ( n = 180) required a blood transfusion. Maternal death has not been reported. Injury to the pregnancy, resulting in spontaneous abortion, intrauterine infection, and preterm birth, however, places the fetus at considerable risk. Rogers and Williams presented a series of 72 pregnancy conizations and reported a perinatal complication rate of 19.4%. Across the literature, the risk of pregnancy loss when the procedure is performed during the first trimester ranges from 15.2% to 33%. Overall, cone biopsy in pregnancy is associated with a 3% to 6% risk of perinatal death as a consequence of profuse hemorrhage or from delivery of a previable or extremely premature fetus through an incompetent cervix. A further point that needs emphasizing is that 30% to 57% of pregnant cones will have dysplasia, microinvasive tumor, or both at the endocervical or ectocervical margins. For this reason, the procedure should not be considered therapeutic in the pregnant patient.
The large-loop electrosurgical excision of the transformation zone (LLETZ) may be used in the operating room to excise a shallow cone of sufficient breadth and depth to permit treatment decisions during pregnancy. Robinson and colleagues reported on 20 women who underwent LLETZ from 8 to 34 weeks of gestational age and noted significant morbidity in patients treated between 27 and 34 weeks of gestational age, including two blood transfusions, three preterm births, and one unexplained intrauterine fetal demise 4 weeks postprocedure. Mitsuhashi and Sekiya performed a LLETZ on nine women during the first 14 weeks of pregnancy, none of whom experienced spontaneous abortion, premature delivery, or excessive bleeding. These preliminary results suggest that LLETZ can be performed safely during the first trimester of pregnancy, but there are insufficient data to determine whether this procedure can replace the traditional cold knife cone biopsy. LLETZ is also associated with a significant proportion of patients left with residual disease.
Hacker and colleagues commented that most authors reserve conization for patients in whom the transformation zone was not fully visualized, microinvasion was shown on biopsy or suspected colposcopically, or possible adenocarcinoma was found on biopsy. If colposcopy is unsatisfactory, one alternative to a full cone is a wedge resection of the cervix, removing only areas incompletely visualized colposcopically. Another option is to place six hemostatic sutures, evenly distributed around the perimeter of the cervix close to the vaginal reflection ( Fig. 12.2 ). These sutures reduce blood flow to the cone bed, evert the squamocolumnar junction, and facilitate performance of a shallow “coin” biopsy with little interruption of the endocervical canal ( Fig. 12.3 ).
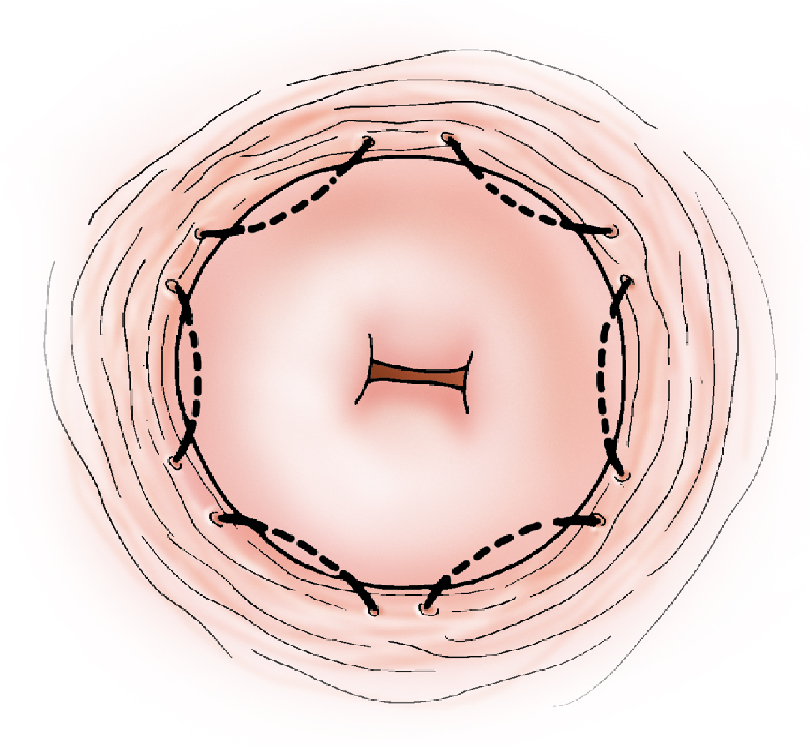
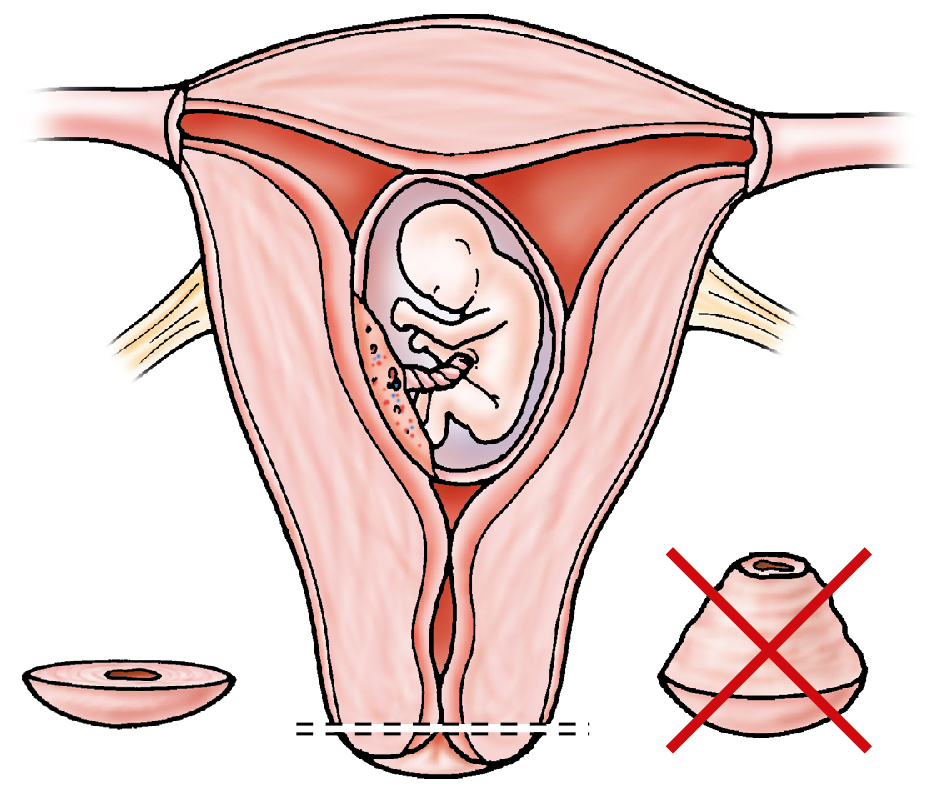
To offset the risk of cervical incompetence, Goldberg and colleagues performed 17 cone cerclages between 12 and 27 weeks of gestation. All procedures were performed with the patient under general anesthesia. After injection of the entire ectocervix with vasopressin (20 units in 60 mL of normal saline), lateral hemostatic 2-0 polyglycolic acid sutures were placed at the 10 and 2 o’clock positions on the cervix, and a standard McDonald cerclage using #1 nylon suture material was inserted as high and as close to the internal cervical os as technically possible without reflection of the bladder. After the cervical cone was excised, the McDonald suture was tied with the knot placed anteriorly, and an iodoform vaginal pack inserted for 24 hours. All 17 patients had uneventful pregnancies, delivering viable infants at or beyond 34 weeks of gestation.
Tsuritani and colleagues have reported on the safety and efficacy of CO 2 laser conization in pregnant women with CIN III or CIS ( n = 30) and MIC ( n = 19). The median gestational age was 17 weeks, and the median length of cervix resected was 14 mm. The median duration of surgery was 20 minutes, and median blood loss was 78 mL. Laser conization identified one case of International Federation of Gynecology and Obstetrics (FIGO) IA2 carcinoma and three cases of FIGO IB1 disease. Of the 35 women who were able to be followed through until delivery, 27 (77.1%) delivered vaginally. Although eight (22.9%) had cesarean sections and six (17.1%) delivered preterm, no CO 2 conization–related obstetric complications were observed.
Fambrini and colleagues have performed CO 2 laser conization in 26 pregnant patients with biopsy-proven CIS or CIN III whose colposcopic evaluation was suspicious for invasion. The procedures were performed during the 18th week of gestation, and no major intraoperative or postoperative complications occurred. Two cases of occult FIGO stage IA1 carcinoma with free surgical margins were diagnosed. Twenty patients (76.9%) delivered vaginally, and six patients underwent cesarean section for indications not related to the prior conization. After a mean postpartum follow-up time of 18 months, 92.3% of patients were cytologically and colposcopically negative for persistent or recurrent disease. Two cases of persistent CIN were managed successfully by re-conization.
Management of cervical intraepithelial neoplasia in pregnancy
The critical issue is to exclude the coexistence of microinvasive disease with pregnancy. This is because everything else, the gamut between cellular atypia and CIS, can be followed expectantly during pregnancy, with treatment deferred after its conclusion. We emphasize the following steps:
- 1.
An experienced colposcopist must accurately assess the disease to determine whether a directed biopsy is indicated.
- 2.
In cases involving a CIS on directed biopsy, a coordinated effort between the gynecologist and the pathologist should be undertaken to determine whether an excisional biopsy is required to exclude invasion.
Management of squamous cell abnormalities
In 2019, the American Society for Colposcopy and Cervical Pathology (ASCCP) updated its recommendations for cervical cancer screening in pregnancy and management of CIN and adenocarcinoma in situ (AIS) in pregnancy. The 2019 guideline recommendations align management recommendations with current understanding of the natural history of HPV and carcinogenesis. Treatment recommendations are based on an individual patient’s risk of CIN 3+ based on current and past results, whereas the previous 2012 guidelines were test results-based algorithms. HPV-based testing (HPV alone or in combination with cytology) is the basis for risk estimation. Cytology alone has lower sensitivity and negative predictive value in estimating long-term risk when compared to HPV-based testing. Personalized risk-based management is possible with knowledge of current results and past history. Management recommendations are based on a combination of past and current results which are used to define the individual’s risk of having or developing CIN 3+. The individual’s risk of CIN 3+, either immediate or 5-year risk, determines the recommended clinical action ( Fig. 12.4 ). The updated guidelines recommend managing abnormal screening results in pregnancy using the same Clinical Action Thresholds established for non-pregnant patients.
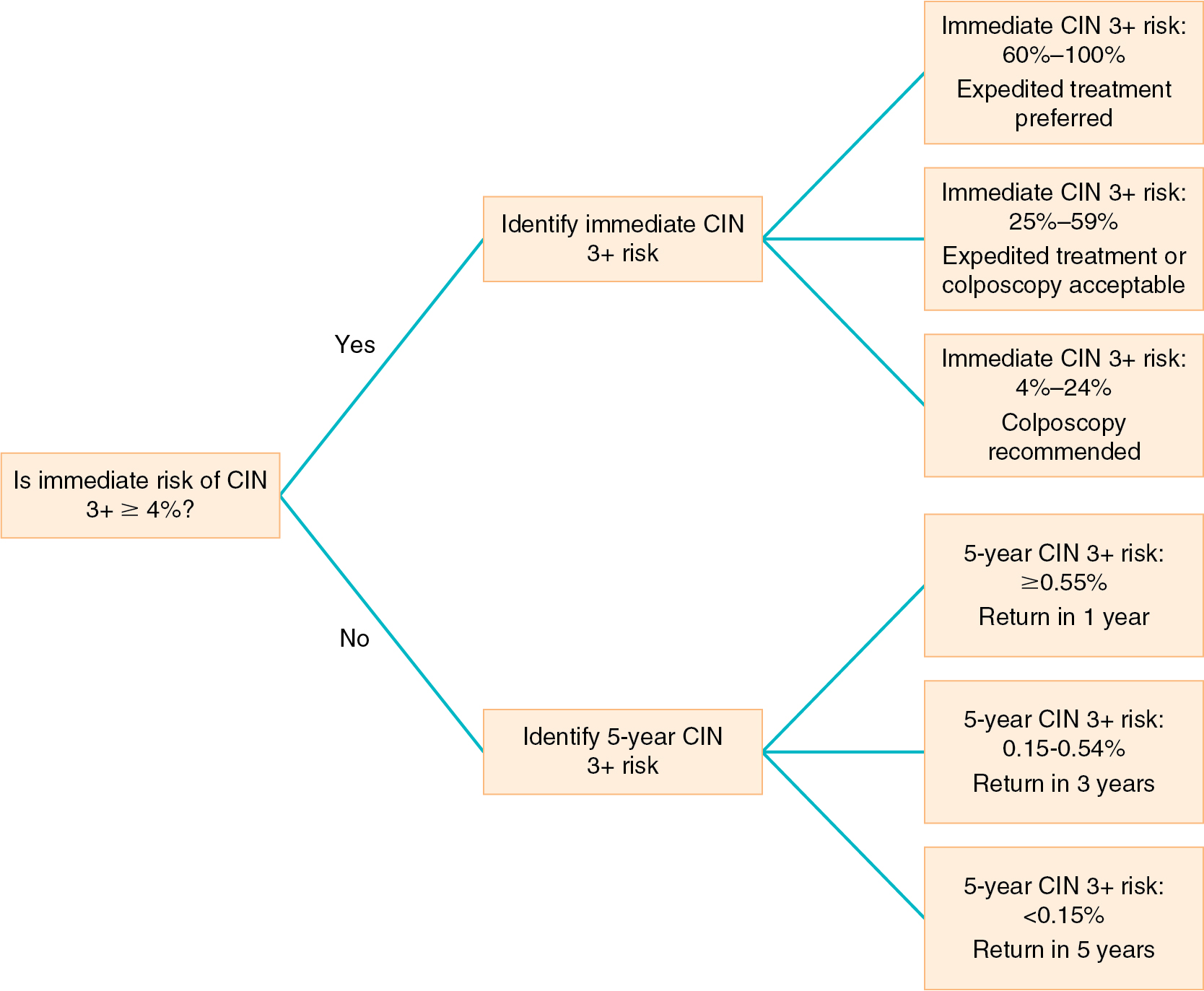
The 2019 guidelines allow for deferral of colposcopy for minor abnormalities to the postpartum period for women with prior negative HPV and no history of CIN 2+. If histologic CIN 2+ is diagnosed at first colposcopy, treatment is not recommended in pregnancy. Close surveillance can be recommended every 12 to 24 weeks with colposcopy or cotesting. Deferring colposcopy to the postpartum period is also acceptable for these patients. On repeat colposcopy, if there is concern for a worsening lesion or microinvasion, biopsy should be repeated.
For pregnant patients diagnosed with CIN 2+ in pregnancy who defer treatment, colposcopy should be performed no sooner than 4 weeks postpartum. If a lesion is still noted, a therapeutic excisional procedure can be performed; repeat cytology, HPV, and biopsy are also acceptable. If a patient was diagnosed with CIN 2+ in pregnancy and there is no lesion on postpartum colposcopy, repeat cytology and HPV testing can be performed.
Patients whose biopsies are suspicious for microinvasion should undergo one of the excisional procedures described previously (i.e., conization or LLETZ with or without cerclage, coin biopsy, or wedge biopsy). If microinvasion is excluded, the patient should be observed during the pregnancy with colposcopy. If microinvasion is established either by directed punch biopsy or by excisional biopsy, recommendations specific for malignant disease must be sought (see later).
In some circumstances, the colposcopic evaluation will be unsatisfactory, in that the entire transformation zone cannot be completely evaluated. If there is no evidence of a severe lesion in the evaluable areas and the original Pap test was not consistent with squamous cell carcinoma (SCC) or AIS, close observation with repeat colposcopy during each trimester of the pregnancy may be considered. Under more dire scenarios, a coin biopsy of the cervix or a wedge biopsy of the hidden part of the transformation zone may be necessary.
Summary of recommendations for squamous cell abnormalities:
- 1.
Observation is preferred to treatment for CIN 1 and expectant management during pregnancy is acceptable for CIN II and III. Diagnostic excisional procedure or repeat biopsy is recommended only if cancer is suspected.
- 2.
In patients for whom there is no concern for microinvasion, serial colposcopy during pregnancy (e.g., every trimester) is not necessary.
- 3.
Definitive management of CIN should be deferred to the postpartum period in most cases.
Management of glandular cell abnormalities
Cervical cytology containing glandular cell abnormalities may be reported as atypical glandular cells not otherwise specified (AGC-NOS), atypical cells favor neoplasia, AIS, or even adenocarcinoma. Of note, 40% of cervices associated with an atypical glandular cells of undetermined significance (AGUS) smear will have a significant tissue abnormality, with greater than 50% harboring an SIL. The significance of an AGUS Pap result in pregnant and in postpartum women is not yet clear. In a recent manuscript by Chhieng and colleagues, 30 pregnant women and 5 within the immediate postpartum window were evaluated for a cytologic diagnosis of AGUS. Of 27 women for whom there was follow-up, 17 underwent colposcopic examination and biopsy. Five women (29.4%) had CIN, including three high-grade and two low-grade lesions on biopsy. It is interesting that the remaining patients (70.6%) had benign pathology, which included chronic cervicitis ( n = 5), endocervical endometrial polyps (or both) ( n = 4), Arias–Stella reaction ( n = 2), and microglandular hyperplasia ( n = 1); of the 10 patients who had repeat Pap tests, only 2 had persistent AGUS or ASC-US. Nevertheless, the finding that up to 30% of patients with pregnancy-associated AGUS had a significant preneoplastic lesion warrants careful evaluation.
The clinician’s diagnostic armamentarium is limited during pregnancy. In the absence of a visible lesion or a significantly expanded cervix (i.e., the barrel-shaped cervix), all patients with AGUS smears should undergo colposcopic evaluation with directed biopsy. Patients diagnosed with AIS should be evaluated by colposcopy during subsequent trimesters. It is not known whether a patient with AIS on directed biopsy should undergo wedge resection of that area to rule out invasion in a nearby “skip” lesion. A diagnostic LLETZ cold knife cervical conization (or both) during pregnancy is best reserved only for those few cases in which an AIS or squamous lesion suspicious for microinvasion is encountered on directed biopsy.
If colposcopy is unrevealing, however, the concern is raised that an endocervical lesion high in the canal or even within the endometrial compartment is being missed. Nevertheless, an endocervical curettage, cervical dilatation with fractional uterine curettage, or endometrial aspiration biopsy is best deferred until the postpartum period, when even a full cervical conization can be performed if needed. Therefore in these clinical scenarios, consultation with the cytopathologist should be arranged to determine if the original cytology contains troublesome features such as inflammatory cells, polyps, glandular hyperplasia, or the Arias–Stella reaction, any of which could confuse the picture, especially when dealing with an AGC-NOS result. Gravid women with a negative colposcopic survey for an atypical glandular cell (AGC) favoring neoplasia cervical smear can be evaluated safely in pregnancy with either endovaginal ultrasonography or MRI of the pelvis to search for a lesion within the endometrium or endocervical canal. In these latter circumstances, referral to a gynecologic oncologist should be contemplated.
The 2019 ASCCP management schema for AIS includes the following:
- 1.
Referral to a gynecologic oncologist is preferable, but management by a general gynecologist experienced in colposcopy and management of AIS is acceptable.
- 2.
Expectant management during pregnancy is acceptable for AIS.
- 3.
In patients for whom there is no concern for microinvasion, serial colposcopy during pregnancy (e.g., every trimester) is not necessary.
- 4.
Definitive management of AIS should be deferred to the postpartum period.
Intrapartum hysterectomy
Some authors have described a program in which an intrapartum hysterectomy (after either vaginal or cesarean birth) is performed for patients with CIS or AIS who have completed childbearing or have proved to be noncompliant. Because there is not sufficient evidence to suggest that an immunocompetent patient is at risk for rapid progression of disease during pregnancy, the need to remove the diseased segment of the cervix is not urgent. Intrapartum hysterectomies, both elective and nonelective, can be associated with significant blood loss. Furthermore, among inexperienced obstetricians, the bladder is particularly at risk for injury. One must balance the noncompliance of a given patient with the possibility that microinvasion may not have been sufficiently excluded during pregnancy, especially in cases of CIS or AIS with positive margins. The observation that postpartum regression may also occur with even CIS argues against the routine performance of an intrapartum hysterectomy for the management of CIN in pregnancy.
Invasive cervical cancer
Presenting symptoms in order of frequency among pregnant women with cervical carcinoma include abnormal vaginal bleeding (63%), vaginal discharge (13%), postcoital bleeding (4%), and pelvic pain (2%). Of importance, in the review by Hacker and colleagues, 18% of patients were asymptomatic, as were 30% of the patients in the study by Creasman and colleagues. When bleeding occurs, this symptom must be investigated and not automatically attributed to the pregnancy. Examination during the first trimester will not lead to abortion. Third-trimester bleeding can be adequately assessed in the operating room as a double setup procedure. Many times, visual inspection is all that is needed for diagnosis of this malignant neoplasm.
In 2018, the FIGO staging system for cervical cancer was updated. For stage IA tumors, lateral extension measurements were removed. Stage IB now has 3 subgroups: ≥5 mm but <2 cm (IB1), ≥2 cm but <4 cm (IB2), and tumors >4 cm (IB3). Additionally, the 2018 FIGO staging includes lymph node assessment by either imaging or surgical pathology. If there is suspicion on imaging or pathologic confirmation of pelvic or para-aortic lymph node involvement, the patient will be up-staged to stage IIIB1 and IIIB2, respectively. The FIGO staging system applies also in pregnancy. To avoid the risks of radiation exposure to a developing fetus, the author recommends ultrasonography of the kidneys to evaluate for the presence of hydronephrosis and an MRI of the pelvis when there is concern for parametrial extension of the tumor. Chest radiography may be performed with appropriate abdominal shielding to exclude pulmonary metastases. An algorithm for the suggested management of invasive cervical cancer in pregnancy appears in Fig. 12.5 .
Halaska and colleagues recently conducted a matched cohort study utilizing the INCIP registry to analyze practice patterns for pregnant women with a diagnosis of cervical cancer. In a cohort of 132 pregnant patients with cervical cancer, the median gestational age at diagnosis was 18.4 weeks, and 14.4% were stage IA, 47.0% were stage IB1, 18.9% were stage IB2, and 19.7% were stage II to IV. Upon diagnosis, 17.4% of patients underwent surgery and 16.7% received neoadjuvant chemotherapy. Of the remaining patients, 26.5% underwent pregnancy termination, 12.9% underwent premature delivery, and 26.5% delayed treatment.
A working group was set up in 2007 in France to propose national recommendations for the management of pregnant patients with invasive cervical cancer. The management of cervical cancer during pregnancy is affected by five factors:
- 1.
FIGO stage (and tumor size)
- 2.
Nodal status
- 3.
Histologic subtype of the tumor
- 4.
Gestational age at diagnosis
- 5.
Patient’s wishes regarding continuation of pregnancy
In patients with early-stage disease diagnosed during the first two trimesters of pregnancy, there is an increasing tendency to preserve the pregnancy while awaiting fetal maturity in patients with absence of nodal involvement.
Microinvasive disease
The diagnosis of MIC in pregnancy is typically established with colposcopic directed biopsy, and in a minority of cases in which the colposcopic biopsy cannot exclude microinvasion, a shallow coin biopsy of the cervix or wedge excision of the area under suspicion as outlined earlier is suggested. This is the only absolute indication for conization during pregnancy. Conization distinguishes patients who have “early stromal invasion” and who can proceed to term without appreciable risk to their survival from those with frank invasion in whom consideration must be given to early interruption of the pregnancy. The author advises patients with early stromal invasion (i.e., FIGO stage IA1) that the pregnancy may continue safely to term, provided the surgical margins are free. Karrberg et al. recently published their 16-year experience from the Western Region of Sweden and emphasize that early detection of cytologic atypia and proper follow-up during pregnancy will likely lead to detection of a high proportion of stage 1 cases, many of which could be cured via cervical conization, thus preserving current and future fertility.
Cesarean section is not thought to be necessary for this group of patients, and the route of delivery should be determined by obstetric indications. Patients with FIGO stage IA2 or occult IB1 lesions should undergo cesarean delivery when fetal pulmonary maturation is demonstrable followed by an immediate modified radical abdominal hysterectomy with bilateral pelvic lymphadenectomies. The author advocates the deployment of a vertical uterine incision so as to leave the lower uterine segment undisturbed for subsequent detailed pathologic examination. It is interesting that the physiologic changes of pregnancy actually enhance the performance of radical surgery by providing the surgeon with multiple levels of distinct tissue planes.
Recently, reports of laser conization for microinvasive disease have originated from Japan. As described earlier, Tsuritani and colleagues have included 19 women with MIC in their series of 49 women and considered CO 2 laser conization within 20 mm of length to be safe in pregnant patients. Yahata and colleagues have reported their experience with four patients diagnosed with stage IA1 cervical adenocarcinoma who underwent KTP (potassium titanyl phosphate) laser conization and vaporization from 16 to 23 weeks of gestation. All patients delivered at term, at which point they underwent radical hysterectomy with lymphadenectomy ( n = 3) or cold knife conization ( n = 1). None have developed recurrent disease during a 2- to 13-year follow-up period.
Cesarean–radical hysterectomy with pelvic lymphadenectomies
In deciding on therapy for frankly invasive cervical cancer in pregnancy, the physician must consider both the stage of disease and the duration of pregnancy. For FIGO stage I and FIGO stage IIA lesions, radical hysterectomy with bilateral lymphadenectomy is acceptable during any trimester ( Fig. 12.6 ). We prefer the surgical approach because of the overall result, which includes ovarian preservation, improved sexual function, and elimination of unnecessary delays for the patient. The complication rate of radical surgery for cervical carcinoma in pregnant patients does not exceed that in nonpregnant patients when normal surgical principles are scrupulously followed.
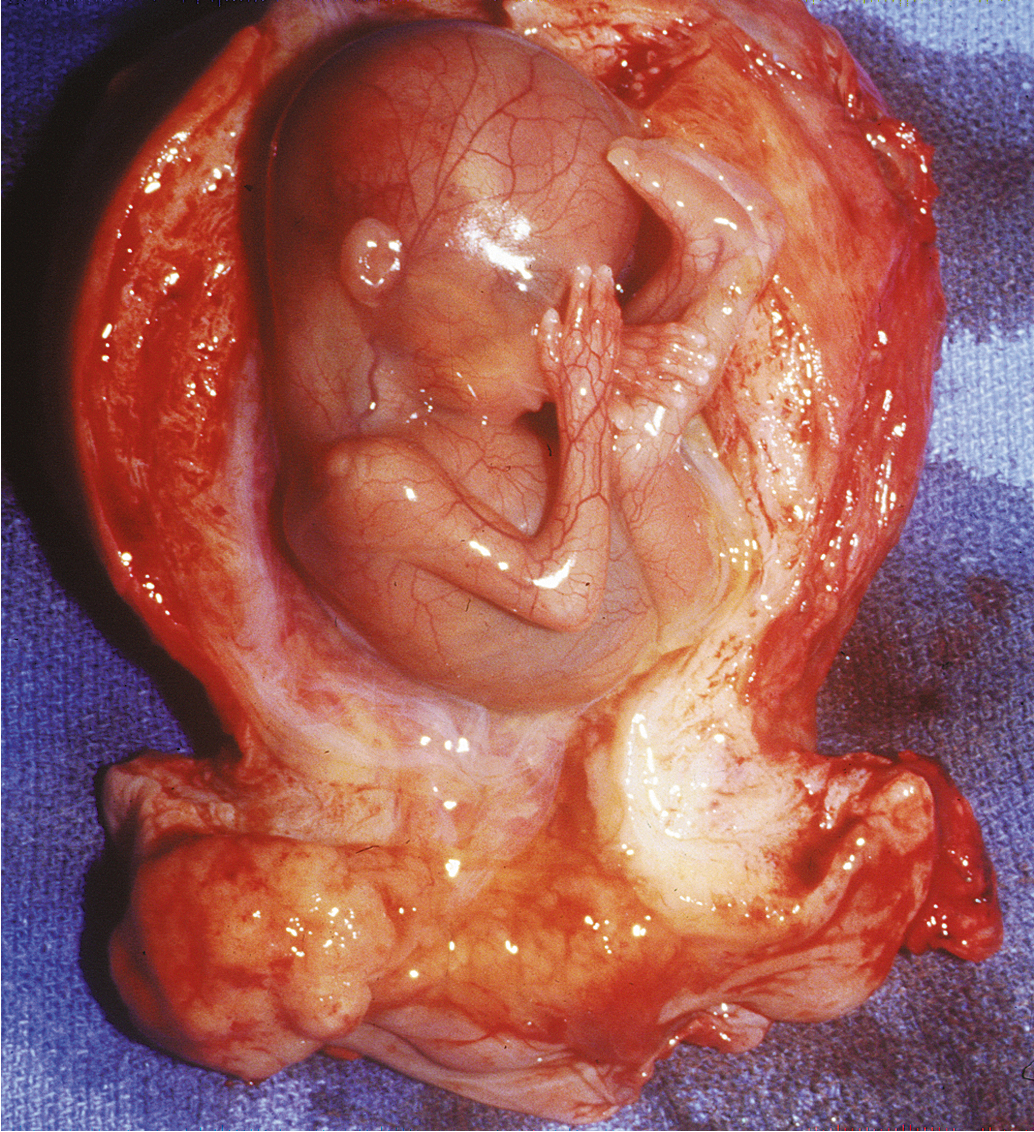
Monk and Montz examined their institutional experience in treating invasive cervical cancer complicating intrauterine pregnancy with radical hysterectomy. They identified 13 patients treated with radical hysterectomy and bilateral pelvic lymphadenectomy with the fetus in situ and 8 others treated with cesarean delivery followed by radical hysterectomy and bilateral pelvic lymph node dissection. The mean operative time was 281 minutes, and the mean blood loss was 777 mL for radical hysterectomy with the fetus in situ plus lymphadenectomy and 1750 mL when cesarean section preceded the cancer operation. The surgical morbidity was minimal for the whole group, and after documentation of fetal maturation, seven healthy infants were delivered. Twenty patients (95%) are alive and free of disease with a mean follow-up of 40 months. The authors concluded that radical surgery offers immediate treatment for early-stage cervical cancer during intrauterine pregnancy, with low associated morbidity, acceptable survival, and preservation of ovarian function.
Radical trachelectomy with lymphadenectomy
In recent years, several reports have appeared describing the successful performance of radical trachelectomy during pregnancy. Abu-Rustum and colleagues treated a 37-year-old pregnant woman at 15 weeks of gestational age for a FIGO stage IB1 poorly differentiated lymphoepithelioma-like cervical carcinoma found on conization. The procedure was performed abdominally and included bilateral pelvic lymphadenectomies and permanent cerclage placement. The final pathology revealed 7-mm (of 19-mm) invasion with no lymphovascular involvement, negative margins, and negative nodes. The pregnancy was delivered by elective planned cesarean hysterectomy at 39 weeks of gestational age. This procedure has also been safely performed during the 19th week of gestation by Mandic and colleagues. Again, the abdominal approach was used, and the patient underwent successful cesarean section at 36 weeks of gestation. At the time of manuscript acceptance, the patient was in the 15th week of a new pregnancy and had normal cytologic screening and no evidence of metastatic or recurrent carcinoma. Finally, Gurney and Blank have described a patient diagnosed with FIGO IB1 disease at 21 weeks of gestational age who underwent postpartum radical abdominal trachelectomy. Ungar and colleagues reported five cases of radical abdominal trachelectomy during pregnancy and the birth of two healthy term infants.
The first case of vaginal radical trachelectomy was reported by van de Nieuwenhof and colleagues to have occurred at 16 weeks of gestation for a FIGO stage IB1 lesion. This patient underwent an uneventful cesarean section followed by radical hysterectomy at 36 weeks of gestational age, and at 9 months of follow-up, both the mother and infant were doing well.
Alouini and colleagues reported laparoscopic pelvic (with and without aortic) lymphadenectomy during pregnancy in eight patients from 12 to 32 weeks of gestation. There were no surgical or general anesthetic maternal or fetal complications. The mean number of lymph nodes removed was 18 (range, 11 to 28), and in five patients (stage IB1, n = 4; stage IB2, n = 1), there was pathologic evidence of nodal metastases. One patient experienced a spontaneous abortion after radical trachelectomy, but the remaining seven reached fetal maturity and had healthy babies by cesarean section. Laparoscopic staging with retrieval of 19 negative nodes for a FIGO stage IB1 carcinoma complicating a twin pregnancy was safely performed at 17 weeks of gestation by Favero and colleagues.
Finally, Silva and colleagues explored the possibility of sentinel lymph node (SLN) mapping in pregnant patients with cervical cancer. They reported the first case of SLN detection using technetium-labeled radiocolloid. Histologic analysis of the operative specimen demonstrated a poorly differentiated squamous carcinoma with metastasis in the sentinel and a neoplastic embolus in a blood vessel of the placental bed.
Whole-pelvis radiotherapy with intracavitary brachytherapy
Radiation therapy is equally efficacious in treating patients with early-stage (i.e., FIGO stage IB1) cervical cancer in pregnancy and, together with radiosensitizing chemotherapy, is the treatment of choice in more advanced stages (FIGO stages IB2–IVA). In the first and second trimesters when the pregnancy is to be disregarded, treatment should begin with whole-pelvis irradiation. Spontaneous abortion usually occurs during therapy, and the treatment is then completed with intracavitary radium or cesium applications. Spontaneous abortion usually occurs at about 35 days in the first trimester and at 45 days in the second trimester after onset of radiotherapy. Some second-trimester patients will go 60 to 70 days before abortion occurs. An alternative approach in the patient who has not aborted is to evacuate the uterus by means of a hysterotomy followed by conventional intracavitary irradiation delivered within 1 to 2 weeks.
If spontaneous abortion does not occur by completion of the external-beam therapy, as occurs commonly after the 16th week of gestation, a modified radical hysterectomy without pelvic lymphadenectomy should be done to excise the remaining central neoplasm. This strategy delivers potentially curative doses of radiation to pelvic lymph nodes with microscopic foci of metastatic tumor followed by surgical resection of the remaining central tumor because the gravid uterus is not suitable for intracavitary radium or cesium. Although some clinicians prefer an extrafascial hysterectomy after 5000 cGy of whole-pelvis irradiation in patients who have early lesions, the author prefers the more extensive modified radical hysterectomy. This approach accomplishes adequate excision of the cervix and accompanying medial parametria and upper vagina, which includes all the tissues that would have been effectively irradiated by the pear-shaped isodose distribution of a tandem and ovoid application of radium or cesium. Those who advocate an extrafascial hysterectomy centrally often advise further vaginal vault irradiation after the surgical procedure to treat the upper vagina and medial parametria more completely.
Sood and colleagues assessed the effects of pregnancy on tumor control, survival, and morbidity associated with radiation therapy administered to pregnant patients. They identified 26 women treated primarily with radiation therapy before the era of concurrent chemoirradiation and matched these patients with 26 control participants based on age, histology, stage, treatment, and year of treatment. Patients were treated with external-beam radiation (mean dose, 46.7 Gy) and intracavitary radiation (mean dose, 56.5 Gy to point A). Three patients diagnosed during the first trimester were treated with radiation with the fetus in situ, and all had spontaneous abortions 20 to 24 days after the start of radiation (mean dose, 34 Gy). In all of these cases, radiation was interrupted for only 3 days or less. There were no statistically significant differences in recurrence rates or survival between the pregnant group and the control participants.
Benhaim and colleagues have reported two patients with locally advanced disease who were treated with chemoradiation during the first trimester with the fetus in utero. The first patient (FIGO stage IVA) was treated at 12 weeks of gestation and experienced recurrence at 20 months. The second patient (FIGO stage IIB) was also treated at 12 weeks of gestation and underwent completion surgery (radical hysterectomy with lymphadenectomy) and has survived disease free for 2 years.
Episiotomy site recurrence
Patients in whom the diagnosis of invasive cervical cancer is made in the postpartum period may have undergone vaginal delivery. This group of patients warrants immediate therapy and specialized surveillance. Recurrence of cervical cancer at the episiotomy scar is a rare event and is thought to occur through implantation at the time of vaginal delivery from an occult tumor, with subsequent early, isolated recurrence as opposed to regional spread. At least 15 cases have appeared in the literature since 1986, including 1 patient who experienced relapse along a perineal laceration scar ( Table 12.4 ). In the majority of these patients, the primary diagnosis of cervical cancer was made during the postpartum period, with recurrence at the episiotomy site typically occurring within 6 months of primary treatment.
| Authors | Year | Stage | Prenatal Cytology | Pathology | Time of Initial Diagnosis | Primary Treatment | Disease-Free Interval | Treatment of Recurrence | Status |
|---|---|---|---|---|---|---|---|---|---|
| Burgess and Waymont | 1987 | IB | SCCA | SCCA | 7 months pp | XRT, BT | 17 months | Exenteration | n/r |
| Copeland et al. | 1987 | IB | Normal | Adenocarcinoma | 3 months pp | RH | 3 months | WE, XRT, BT | NED >5 years |
| IB | Normal | Adenocarcinoma | Biopsy at delivery | RH | 5 months | WE, XRT | NED 10 months | ||
| Gordon et al. | 1989 | IB | Normal | SCCA | Biopsy at delivery | RH | 1 month | WE, XRT, BT | NED 3.5 years |
| van Dam et al. | 1992 | IIIA | n/r | SCCA | 6 weeks pp | Chemotherapy, XRT, BT | 0 months | BT | n/r |
| IB | Normal | Adenocarcinoma | 9 weeks pp | XRT, BT | 3 months | WE, XRT, BT | NED >10 years | ||
| Khalil et al. | 1993 | IIIB | Not done | SCCA | 8 weeks pp | XRT, BT | 3 months | Chemotherapy, XRT | DOD 4 months |
| Cliby et al. | 1994 | IB | HSIL | SCCA | Biopsy at delivery | RH | 9 weeks | Chemotherapy | DOD 6 months |
| IB | Not done | SCCA | Biopsy at 36 weeks | RH | 24 months | Chemotherapy, WE, XRT | NED 1 year | ||
| IB | Not done | SCCA | Biopsy at delivery | RH | 3 months | XRT | DOD 3.5 years | ||
| IB | Not done | SCCA | 5 weeks pp | RH | 1 months | WE, XRT | DOD 6 months | ||
| van den Broek et al. | 1995 | IA1 | Normal | Adenocarcinoma | 3 months pp | Total abdominal hysterectomy | 6 weeks | WE, interstitial implants | DOD 1 years |
| Goldman and Goldberg | 2003 | IB | Normal | SCCA | 1 weeks pp | RH | 5.5 years | WE, XRT, BT | DOD 4.5 years |
| Heron et al. | 2005 | IB | AGUS | Villoglandular | Biopsy at 31 weeks | RH, BPLND | 44 months | XRT, WE | NED 10 months |
| Baloglu et al. | 2007 | IIIA | Unknown | SCCA | 8 months pp | ChemoRT | NED 1 year f/u |
In a matched case-control study of women with cervical cancer diagnosed during pregnancy ( n = 56) or within 6 months of delivery ( n = 27), Sood and colleagues noted that among the patients diagnosed postpartum, 1 of 7 who was delivered by cesarean section developed a local and distant recurrence, but 10 of 17 (59%) of those who delivered vaginally developed recurrences ( P = .04). In multivariate analysis, vaginal delivery was the most significant predictor of recurrence. We recommend delivery by cesarean section when the diagnosis is known antenatally, and in patients diagnosed in the postpartum period, vigilant examination of the episiotomy and vaginal laceration sites is warranted. Although the mode of delivery in the setting of known microinvasive disease may be based on obstetric indications, it should be pointed out that among the 14 cases of episiotomy site recurrences, there was one patient who had been diagnosed with a stage IA endocervical adenocarcinoma.
Neither the time of diagnosis nor time to recurrence appears to affect survival after episiotomy site recurrence. Goldman and Goldberg noted that no patient who received chemotherapy or radiotherapy for recurrent disease without excision survived 1 year. The treatment policy should include wide local excision of the entire nodule with adjuvant external radiotherapy plus brachytherapy. Of seven patients treated by this method, 71% were without evidence of disease at longer than 1 year.
There have been at least two cases of cervical cancer with concomitant episiotomy metastasis in the literature. Baloglu and colleagues reported one of these patients for whom the diagnosis was unknown antenatally. This patient was diagnosed with locally advanced disease and an episiotomy site metastasis and received primary chemoradiation. She was without evidence of recurrence at 1 year of follow-up when the report was published. Women diagnosed with cervical cancer in the postpartum period should have the episiotomy or vaginal laceration site carefully examined if they experience vaginal delivery.
Planned delay of therapy
Historically, when invasive carcinoma was diagnosed before 20 weeks of gestation, recommendations included immediate treatment of the tumor, either by radical hysterectomy or radiation therapy, leaving the fetus in utero in both instances. This dogma has been challenged during the preceding decade, with multiple reports of a safe outcome for mother and child with a deliberate delay in therapy to permit gestational advancement ( Table 12.5 ). For example, Duggan and colleagues reported a mean diagnosis-to-treatment interval of 144 days (range, 53 to 212 days) in eight patients with FIGO stage IA or FIGO stage IB cervical cancer who postponed therapy to optimize fetal outcome. All of these women were rendered disease free after a median follow-up period of 23 months. Sorosky and colleagues identified eight pregnant women with FIGO stage I squamous cell cervical carcinoma who declined immediate therapy to improve fetal outcome. They were observed prospectively until the late third trimester, with a mean diagnosis to treatment interval of 109 days (range, 21 to 210 days). No clinical progression of disease was detected, and after therapy, all were alive and disease free after a mean follow-up time of 37 months (range, 13 to 68 months). Takushi and colleagues reported a delay in treatment of 6 to 16 weeks for four women with FIGO stage IA2, IB1, or 1B2 lesions. No disease progression was documented, and after cesarean–radical hysterectomy, all patients have been disease free at a follow-up period of 70 to 156 months.
| Authors | Year | Stage | Patients ( n ) | Delay | Maternal Outcome |
|---|---|---|---|---|---|
| Prem et al. | 1966 | I | 4 | 6 weeks | NED 5 years |
| I | 5 | 11–17 weeks | NED 3–5 years | ||
| Dudan et al. | 1973 | IB | 2 | 2 and 6 months | Progression |
| Lee et al. | 1981 | IB | 1 | 12 weeks | NED 10 years |
| IB | 2 | 11 weeks | No progression | ||
| II | 5 | 1–11 weeks | No progression | ||
| Nisker and Shubat | 1983 | IB | 1 | 24 weeks | DOD |
| Greer et al. | 1989 | IB | 5 | 6–17 weeks | NED 1–3 years ( n = 4), DOD ( n = 1) |
| Monk and Montz | 1992 | IB | 4 | 10–16 weeks | NED 3.5 years |
| Hopkins and Morley | 1992 | IB | 5 | 12 weeks | NED 5 years ( n = 40) |
| Mack et al. | 1981 | IB | 3 | 10–16 weeks | NED |
| Duggan et al. | 1993 | IB1 | 5 | 7–24 weeks | NED 3 years |
| Sivanesaratnam et al. | 1993 | IB | 2 | 2 and 4 weeks | NED 5 years |
| Allen et al. | 1995 | IB | 2 | 18–19 weeks | NED 5 years |
| Sorosky et al. | 1995 | IB1 | 7 | 7–29 weeks | NED 1.5–5.5 years |
| Sood et al. | 1996 | IB | 3 | 3–32 weeks | NED 1–30 years |
| Tewari et al. | 1997 | IB2 | 1 a | 11 weeks | NED 2 years |
| IIA | 1 a | 18 weeks | DOD 9 months | ||
| van Vliet et al. | 1998 | IB | 5 | 2–10 weeks | DOD ( n = 1), NED 1.5–9 years |
| IIA | 1 | 2 weeks | NED 12 yeasr | ||
| Marana et al. | 2001 | IIB | 1 a | 21 week | DOD 18 months a |
| Takushi et al. | 2002 | IB1 | 2 | 13 and 15 weeks | NED 8 and 9 years |
| IB2 | 1 | 6 weeks | NED 7 years | ||
| Germann et al. | 2005 | IB1 | 9 | 4 months | NED 5 years |
| Traen et al. | 2006 | IB1 | 1 | 19 weeks | NED |
| Gonzalez Basquet et al. | 2008 | IB | 1 | 8 weeks | NED 1 year |
| Favero et al. | 2010 | IB1 | 10 | 11–28 weeks | NED 5-102 months |
| IB2 | 1 | 12 weeks | NED 68 months |
Although most patients with FIGO stage IB disease have fared well with deliberate delays in therapy, four patients are noted in whom progression of disease was observed ( n = 2) or in whom recurrence and death from disease occurred ( n = 2). Five patients with FIGO stage II disease who opted to delay therapy were reported by Lee and colleagues, and although they did not progress during the pregnancy, it should be noted that their cancers were diagnosed in the third trimester, and the treatment delays were relatively limited. Long-term follow-up data were not presented for this subset of patients. Thus, for patients with FIGO stage IA1 to IB1 squamous cell lesions diagnosed before and after 20 weeks of gestation, a limited treatment delay to await fetal maturity may be acceptable. The counseling in such situations should be analogous to obtaining an informed consent from the mother.
Neoadjuvant chemotherapy in pregnancy
Patients with advanced disease (i.e., FIGO stage IB2 and greater) should be offered immediate therapy. A novel approach to patients with locally advanced disease who declined interruption of pregnancy was first reported by our group in 1998. Two women with FIGO stage IB2 and FIGO stage IIA lesions refused interruption of their pregnancies and received neoadjuvant chemotherapy consisting of vincristine (1 mg/m 2 ) and cisplatin (50 mg/m 2 ) during the early second and third trimesters. Both patients experienced significant tumor regression, rendering radical hysterectomy feasible at the time of cesarean delivery after documentation of fetal pulmonary maturation at 32 weeks and 34 weeks of gestation. At the time of publication, one patient had remained without evidence of recurrence for more than 2 years; unfortunately, the second patient experienced a lethal relapse 5 months after primary therapy. Both children have experienced normal development. Another early report was by that of Marana and colleagues, who treated a pregnant woman with a FIGO stage IIB tumor with bleomycin (30 mg on day 1) and cisplatin (50 mg/m 2 on day 2 and day 3) from 17 weeks to 38 weeks of gestation and achieved both tumor regression and a healthy infant, who continued to thrive long after the mother succumbed to recurrent disease 13 months after delivery. Although such a treatment approach remains investigational, the use of neoadjuvant chemotherapy while awaiting gestational advancement may be entertained when the pregnant woman with cervical cancer, for whom a treatment delay is ill advised, refuses interruption of therapy.
Since these initial publications, there have been at least eight additional case reports of patients who received neoadjuvant chemotherapy during pregnancy for cervical cancer along with a recent 21-patient case series reported by Kohler et al. Most patients were treated for locally advanced disease (FIGO IB2–IIIB), but two patients received neoadjuvant chemotherapy for FIGO stage IB1 lesions. Neoadjuvant therapy was usually administered during the mid-second trimester to the early third trimester (range, 17 to 33 weeks of gestation) and single-agent cisplatin (e.g., 75 mg/m 2 every 10 days) has been used most often. The combination of cisplatin (50 mg/m 2 ) plus vincristine (1 mg/m 2 ) every 21 days has also been of continued interest in some cases. Obstetric outcomes have been universally favorable, as have maternal outcomes, although there was one case of rapid tumor progression. It should be emphasized, however, that despite the good results, follow-up in several cases has been of short duration.
In the aforementioned study by Kohler et al., 21 pregnant women at a mean gestational age of 17 weeks (range, 13 to 23 weeks) received a median of three cycles (range, two to four cycles) of platinum-based chemotherapy with cesarean delivery carried out between 30.4 and 36.5 weeks of gestation, resulting in 22 healthy babies without renal, hepatic, auditory, or hematopoietic impairment. Platinum concentrations in the umbilical cord blood and amniotic fluid were 23% to 65% and 11% to 42%, respectively. Because the observed in vivo measurement of platinum was consistently lower in the fetoplacental unit, the authors suggest that a placental filtration mechanism of platinum may exist.
In a meta-analysis by Song et al., 88 pregnant women receiving neoadjuvant chemotherapy were identified. Most were diagnosed during the second trimester (84.8%) with stage I–IIA (87.5%) disease. All but two patients received cisplatin (55 single-agent; 31 combined with paclitaxel, vincristine, doxorubicin, 5-FU, or bleomycin). The majority had a complete (8.7%) or partial response (46.4%), 42.0% experienced stable disease, and only 2.9% progressed. All were delivered via cesarean (79% [ n = 65] by cesarean-radical hysterectomy), except for one patient who presented at 33.1 weeks with advanced cervical dilatation. Ultimately, 84 pregnancies resulted in 88 live-born infants, 71 of whom were completely healthy. Seventeen experienced respiratory difficulty ( n = 9), anemia ( n = 2), and there was 1 case each of mild elevation of serum creatinine, hypoglycemia, first degree intraventricular hemorrhage (IAH), bilateral hearing loss, erythema, and supraventricular tachycardia. Follow-up was noteworthy for one case of acute myeloid leukemia (AML) diagnosed at 22 months and rhabdomyosarcoma at 5 years. A relationship between alkylating agents and leukemia has been previously reported, but proof of direct causality remains elusive. In this case, the neonate lacked chromosomal translocations typical of secondary AML and the karyotypic abnormalities observed in treatment-related cancers.
Neoadjuvant chemotherapy should be considered for patients with locally advanced disease diagnosed in the early to mid-second trimester who are adamant about continuing the pregnancy. Again, these patients need to be counseled regarding the investigational nature of this treatment modality under the clinical circumstances in question.
Prognosis for patients with cervical cancer in pregnancy
The overall prognosis for all stages of cervical cancer in pregnancy is similar to that in nonpregnant women ( Table 12.6 ). The favorable overall prognosis for pregnant patients is related to a greater proportion of pregnant patients with stage I disease. In a report by Allen and colleagues of 96 cases of cervical cancer occurring in pregnancy, the disease-free survival rates for 87 patients who were available for analysis were noted to be 92.3% for FIGO stage IA1, 68.2% for FIGO stage IB, 54.5% for FIGO stage II, and 37.5% for FIGO stage III. The overall survival rate was 65.5%, which is slightly better than that reported by Hacker and colleagues. They also observed an association of advanced clinical staging with diagnosis in the third trimester and postpartum. Of 49 cases of FIGO stage IB cervical carcinoma, 64.5% were diagnosed in the third trimester and postpartum; of 22 cases of FIGO stage II cervical carcinoma, 77.3% were diagnosed in the third trimester and postpartum; and all nine cases of stage III cervical carcinoma were diagnosed in the third trimester and postpartum. Of the 32 patients who underwent pelvic lymphadenectomy, 10 were noted to have positive nodes. This increase in frequency has not been our experience.
| Year | Cases ( n = 41) | Control Participants ( n = 82) |
|---|---|---|
| 0 | 1.0 | 1.0 |
| 2 | 0.89 | 0.87 |
| 4 | 0.86 | 0.79 |
| 6 | 0.82 | 0.75 |
| 8 | 0.81 | 0.73 |
| 10 | 0.79 | 0.73 |
| 12 | 0.77 | 0.73 |
Zemlickis and colleagues compared 40 women who had carcinoma of the cervix in pregnancy with 89 nonpregnant women matched for age, stage, and tumor type. Long-term survival rates were similar between the two groups. When pregnant women were compared with a series of 1963 cervical cancers in women younger than 45 years treated during the same time, the pregnant women were three times more likely to have stage I disease and had a lower chance of having FIGO stage III to IV cancers.
In 1993, Sivanesaratnam and colleagues reported surgical management of early invasive cancer of the cervix in a series of 18 patients who underwent radical hysterectomy and pelvic lymphadenectomy, with a 5-year survival rate of 77.7%. A comparable group of nonpregnant patients who also underwent radical surgery had a survival rate of 92.3%. Nisker and Shubat also reported that there was a slightly better survival in the nonpregnant group than in the pregnant group. These reports are in contrast to the previous reports by Creasman and colleagues, Sablinska and colleagues, and Lee and colleagues, who found no appreciable difference in the 5-year survival rates of pregnant versus nonpregnant patients with cervical cancer.
A multicenter, retrospective study conducted by the Korean Gynecologic Oncology Group (KGOG-1006) contained 40 pregnant subjects treated from 1995 to 2003. Each case was matched to three control participants on the basis of age, stage, histology, and date of treatment. Among 12 patients who delayed treatment for fetal maturity, 2 died of disease. There was no difference in overall survival between pregnant and nonpregnant patients with stage IB lesions.
Pettersson and colleagues recently published a 90-year experience from the Radiumhemmet. The 10-year actuarial survival rate improved significantly during the study period from 27% (1914 to 2004) to 79% (1960 to 2004). The 10-year cause-specific cumulative actuarial survival rate for 41 pregnant women treated during 1960 to 2004 did not differ statistically from the rate for an age-matched, stage-matched, and histopathology-matched control series of nonpregnant women treated at the Radiumhemmet during the same period. The authors concluded that during the study period, the incidence of cervical cancer during pregnancy declined, the cases were discovered at earlier stages, and survival improved.
For more advanced disease, pregnancy may have an unfavorable effect on prognosis as a result of problems with radiation dosimetry in pregnancy and the need to interrupt radiation therapy more frequently because of genital tract sepsis. Clinical stage remains the most important determinant of prognosis.
Obstetric outcomes
Dalrymple and colleagues analyzed the obstetric outcomes among women in California with pregnancy-associated cervical cancer. Using computer-linked infant birth and death certificates, discharge records, and cancer registry files, cases were identified and then assigned to a prenatal or postpartum cancer diagnosis group. Among 434 cases, those diagnosed prenatally ( n = 136) had higher rates of cesarean section, hospitalization longer than 5 days, low birth weight, very low birth weight, prematurity, and fetal deaths compared with pregnant control participants without cancer. No neonatal deaths were attributable to elective premature delivery. Very low birth weight, prematurity, and fetal death rates remained elevated among those diagnosed postpartum.
Key summary points for the management of invasive cervical cancer during pregnancy:
- 1.
Reports describing the successful performance of trachelectomy during pregnancy, occasionally using robotic surgical platforms, continue to appear in the literature for management of early-stage disease.
- 2.
For patients with locally advanced disease who insist on a definitive treatment delay to allow gestational advancement with concomitant fetal maturation, platinum-based neoadjuvant chemotherapy has been reported in more than 80 cases without significant maternal or fetal sequelae despite the observation that platinum does cross the maternal–fetal placental interface.
Ovarian cancer
Ovarian cancer is reported to occur in 1 per 10,000 to 1 per 25,000 pregnancies. Pregnancy does not alter the prognosis of most ovarian malignant neoplasms, but complications such as torsion and rupture may increase the incidence of spontaneous abortion or preterm delivery. In a survey by Kohler of the largest studies in the literature, about 1 in 600 pregnancies will be complicated by an adnexal mass. More contemporary accounts suggest that adnexal masses may complicate as many as 1 in 190 pregnancies. At least one-third of pregnant women are asymptomatic, with the adnexal mass often discovered during obstetric ultrasonography.
Most cysts in pregnant patients are follicular or corpus luteum cysts and are usually no more than 3 to 5 cm in diameter. Functional cysts as large as 11 cm in diameter have been reported but are rare. More than 90% of these functional cysts will disappear as pregnancy progresses and are undetectable by the 14th week of gestation. It appears that the size of the adnexal mass at the time of diagnosis is inversely related to the likelihood of spontaneous regression. Only 6% of masses smaller than 6 cm persisted during serial examinations, but 39% of masses larger than 6 cm persisted. The complication rate increases with increasing size of the mass. In addition, a solid or complex ultrasonographic appearance and the presence of bilateral adnexal or ovarian abnormalities may also be indications to proceed with laparotomy. Adnexal masses with blood flow characterized by a high resistive index by Doppler ultrasonography are less likely to be malignant, independent of size. MRI may be useful when ultrasonographic findings are equivocal.
The most pressing problems associated with ovarian tumors in pregnancy are the initial diagnosis and the differential diagnosis. When the tumor is palpable within the pelvis, it must be differentiated from a retroverted pregnant uterus, a pedunculated uterine fibroid, a carcinoma of the rectosigmoid, a pelvic kidney, and a congenital uterine abnormality (e.g., rudimentary uterine horn). Analysis of serum tumor markers is a complex undertaking and can be misleading because the titers for each of the markers, especially α-fetoprotein and β-human chorionic gonadotropin (hCG), and even cancer antigen 125 (CA-125), are routinely elevated in pregnancy for reasons unrelated to malignancy.
A proposed management algorithm for the adnexal mass in pregnancy appears in Fig. 12.7 . Our experience has been that patients operated on around the 18th week of gestation have negligible fetal wastage associated with the exploration. Therefore, 18 weeks of gestation appears to be a judicious period for laparotomy in terms of its safety both for the fetus and for the elimination of functional ovarian cysts. If the cyst is complex and suspicious for malignancy and increases in size, the patient should undergo exploration earlier than 18 weeks. Whenever exploration is conducted, our recommendation is that the uterus not be manipulated during surgery (i.e., the so-called hands-off-the-uterus approach) in an effort to minimize its irritability.
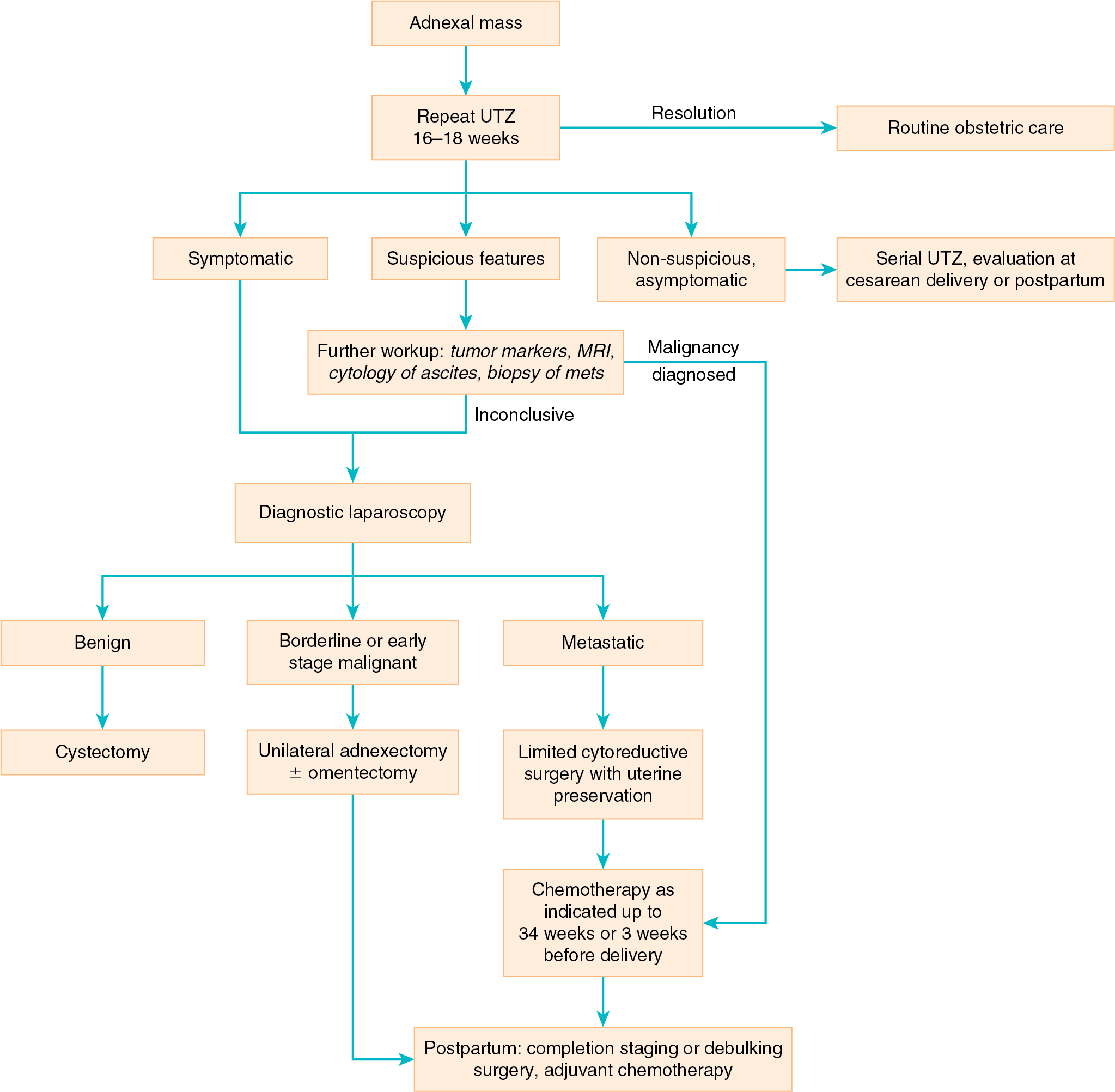
Torsion is common in pregnancy, with 10% to 15% of ovarian tumors reportedly undergoing this complication. Most torsions (i.e., 60%) occur when the uterus is rising at a rapid rate (8 to 16 weeks) or when the uterus is involuting (in the puerperium). The usual sequence of events is sudden lower abdominal pain; nausea; vomiting; and in some cases, shock-like symptoms. The abdomen is tense and tender, and there is rebound tenderness with guarding. If exploration must be undertaken during the first trimester and extraction of the ovary (or ovaries) is required, supplemental progesterone can be administered to decrease the likelihood of pregnancy loss.
In many instances, the presence of an ovarian tumor may not be suspected until delivery ( Fig. 12.8 ). The large uterus obscures the growth of the ovarian neoplasm. The tumor may be growing in the abdomen behind the large uterus and may not fall back into the cul de sac until it is large. If there is a mechanical obstruction of the birth canal, exploratory laparotomy is indicated for both delivery of the baby and management of the ovarian neoplasm. Allowing labor to proceed when an ovarian neoplasm is causing obstruction of the birth canal may result in rupture of the ovarian cyst. Even if the cyst is not ruptured, the trauma of labor may cause hemorrhage into the tumor followed by necrosis and suppuration.

Asymptomatic adnexal masses
Before detailing the clinical approach to managing the different ovarian malignancies that may occur during pregnancy, some consideration should be given to asymptomatic adnexal masses. Admittedly, asymptomatic masses can be malignant; however, the vast majority are likely to be benign, particularly in the pregnant population.
Endovaginal pelvic ultrasonography is essential in the evaluation of adnexal masses. Approximately 10% of masses are complex, and the examination should determine the origin of the mass and its location, size, and internal structure (e.g., vegetations, septations). The mass should be classified unilocular, unilocular-solid, multilocular, multilocular-solid, or solid. Color Doppler imaging may be used to obtain a vascular road map of an ovarian mass. Pelvic MRI with gadolinium injection can be performed after the first trimester and should only be used during pregnancy to remove further doubt regarding possible malignancy or to provide additional information if ultrasonography is inconclusive.
There is no consensus on management of adnexal masses simply based on size. In the general population, the American College of Obstetricians and Gynecologists (ACOG) identifies adnexal masses greater than 10 cm as concerning for malignancy; however, simple and asymptomatic cysts can be expectantly managed. Historically, surgery was considered for asymptomatic masses greater than 6 cm due to the risk of torsion and concern for malignancy. Koo et al. studied 470 pregnant women and reported that masses between 6 and 10 cm are nearly three times more likely to cause torsion than those less than 6 cm. Masses greater than 15 cm had a 12-fold higher risk of malignancy than those less than 6 cm, while masses 6 to 15 cm were not more likely to be malignant compared to those less than 6 cm.
Many masses can be followed conservatively in the absence of symptoms and in the absence of concerning sonographic ovarian and extraovarian findings (e.g., ascites). However, if a patient with an adnexal mass is expectantly managed, they should be counseled on the risk of intermittent versus complete torsion (10%), rupture (2%), or occult malignancy (1% to 9%).
Surgical management of the ovarian mass
Laparoscopy was previously thought to be contraindicated in pregnancy because of the unknown effect of the pneumoperitoneum on the gravid uterus, the possible injection of carbon dioxide into the amniotic cavity, and the potential for acidosis in the fetal environment as a result of maternal conversion of carbon dioxide into carbonic acid. Growing evidence, however, suggests that laparoscopy is not only safe in pregnancy, but is preferred to laparotomy.
Laparoscopy can be safely performed during pregnancy, with most surgeons supporting minimally invasive procedures between 16 and 20 weeks of gestation. There have been reports of laparoscopy up to the 28th week of gestation, but this appears to be the upper limit. Larger uteri increase surgical difficulty, and surgery after 23 weeks can be linked to adverse fetal outcomes and preterm labor. Intervention before the second trimester is not advisable because this does not give time for the ovarian mass to resolve on its own and could compromise ovarian hormone production before the placenta is fully functional.
The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) published guidelines in 2017 for laparoscopic surgery during pregnancy. The patient should be tilted in the left lateral decubitus position to minimize compression of the inferior vena cava and ensure adequate venous return. Initial abdominal entry in the left upper quadrant or subxiphoid region should be used with at least 6 cm between the point of entry and the top of the fundus. The open Hasson technique is preferred over the Veress technique, although both have been used successfully in pregnancy. Ultrasound guidance may be used with trochar insertion with elevation of the abdominal wall to reduce the risk of uterine injury. The table should be tilted to the left or the right with the patient supine to move the uterus away from the site of subsequent trocar insertion.
Intraabdominal pressure should be kept between 10 to 15 mm Hg with the patient in the Trendelenburg position to ensure adequate venous return and uteroplacental blood flow during the operation. Positioning in this manner may require positive pressure ventilation to maintain adequate lung volumes. Depending on the circumstances and gestational age, fetal monitoring may be advisable.
Robotic-assisted laparoscopic management of adnexal masses during pregnancy
With widespread adoption of robotic surgical platforms for management of adnexal masses and endometrial cancer in the nonpregnant population, it is not surprising to see reports of robotic surgical applications applied to the pregnant patient. Eichelberger et al. evaluated their experience in planned robotic resection of adnexal masses in 19 pregnant women, all of which were performed without fetal or maternal complications. When compared with 50 consecutive laparoscopic cases from a recent historical database, the authors noted no differences in operative time, conversion to laparotomy, intraoperative or postoperative complications, or negative observed obstetric outcomes. Interestingly (and not altogether unexpected given the reported advantages of robotics in the nonpregnant population), in this study, the patients undergoing robotic surgery had a significantly shorter length of hospital stay and estimated blood loss. We anticipate surgeons continuing to report further experiences with robotic pelvic surgery in the coming years.
Ovarian masses specific to pregnancy
Two adnexal conditions may specifically be associated with pregnancy. The operating surgeon must be cognizant of their possibility so that unnecessary oophorectomies will not be performed. The luteoma of pregnancy can vary in size from microscopic to 20 cm in diameter and usually consists of multiple, well-circumscribed nodules that can be bilateral in one-third of cases. The luteoma may be associated with significant elevations in plasma testosterone and other androgens in about 25% of the cases. Maternal hirsutism or virilism may occur during the latter half of pregnancy, which may cause virilization in up to 70% of female infants born to masculinizing mothers. If the lesion is not recognized grossly, a biopsy may be taken for definitive diagnosis. Because these regress spontaneously postpartum, nothing further needs to be done. Theca-lutein cysts may occur when hCG concentrations are abnormally elevated, such as in a molar pregnancy, fetal hydrops, or multiple gestations. These are usually multiple and thin-walled cysts. Sometimes, massive bilateral theca-lutein cysts manifest that are considerably different from the solid nodules of the luteoma of pregnancy. These also regress postpartum and should not be resected unless acute complications develop.
Histologic types of ovarian tumors
Thornton and Wells reviewed 131 ovarian enlargements in pregnancy, 81 of which were removed (including 1 carcinoma and 6 borderline lesions). Thirty-nine were greater than 5 cm in diameter and had simple internal echo patterns and smooth walls; three of these were borderline malignant neoplasms. Hoffman reviewed 13 reports of ovarian neoplasms removed in pregnancy and found benign cystadenomas or cystic teratomas most frequently diagnosed. The Hoffman review also included a summary of 127 malignant ovarian lesions found during pregnancy. A recent literature review by Kwon and colleagues describing the various histologies of ovarian tumors found in pregnancy appears in Table 12.7 . Borderline and frankly malignant epithelial lesions were the most commonly encountered during pregnancy.
| Histology | (%) | (%) | (%) | PUMCH, 2003 (%) | (%) | (%) |
|---|---|---|---|---|---|---|
| Epithelial—borderline | 65 | 37.5 | 33–40 | 50 | 39.1 | 81.5 |
| Epithelial—invasive | 35 | — | 66 | 27.3 | 21.7 | 55.5 |
| Germ cell | 17 | 45 | 30–33 | 40.9 | 47.8 | 18.5 |
| Sex cord–stromal | 13 | 10 | 17–20 | 9.1 | 13 | 0 |
| Other | 5 | 7.5 | 12–13 | 0 | 0 | 0 |
Borderline ovarian tumors
Adnexal masses larger than 6 cm that persist into the second trimester warrant removal at approximately 18 weeks of gestational age. Between 2% and 5% of these lesions will be malignant, with dysgerminoma being most common. Among epithelial tumors, serous carcinoma and serous tumors of low malignant potential are readily encountered. In the case series presented by Fauvet and colleagues, 40 patients with borderline ovarian tumors in pregnancy were evaluated. Thirty-six of the 40 patients underwent surgery in pregnancy—10 in the 1st trimester, 22 in the 2nd trimester, and 4 at the time of cesarean. Laparotomy was performed more commonly than laparoscopy, and the majority underwent unilateral salpingo-oophorectomy. The majority of patients were FIGO stage I at initial surgery (31 stage IA, 2 stage IB, 2 stage IC). Restaging surgery was performed in 21 patients, and 5 were upstaged as a result.
Recent evidence suggests that the hormonal influence of pregnancy can lead to histologic changes in serous low malignant potential tumors that, if not sorted out and characterized appropriately, could be mistaken for frankly invasive carcinoma. The group at the MD Anderson Hospital and Tumor Institute collected 10 cases from 1944 to 1993 and conducted a slide review, noting some very peculiar histologic features distinct from those seen in nonpregnant patients, including epithelial atypia and proliferation, eosinophilic cells, mucin production, decidual changes, and frequent microinvasion. Although these lesions remained within the spectrum of low malignant potential tumors, the histologic features were worrisome for a more aggressive clinical course, yet all 10 patients remained disease free after a variety of treatment modalities. We advise prompt recognition of these histologic findings after cystectomy or oophorectomy during pregnancy so as to classify them accordingly as being borderline rather than to confuse them with low-grade serous papillary carcinomas. Of additional interest is that in two cases, the tumor was resected both during pregnancy and after parturition (2 months and 3 years), and there was significant regression of the epithelial proliferation, the number of eosinophilic cells, and the amount of mucin production the second time around; this regression after parturition supports a hormonal etiology of these unusual histologic features. In contrast to frankly malignant ovarian carcinomas, unilateral adnexectomy is all that is required during pregnancy for the serous low malignant potential tumor.
Frankly malignant ovarian tumors
Malignant ovarian tumors account for only 2% to 5% of all ovarian neoplasms found in pregnancy. The incidence for all pregnancies is 1 in 8000 to 1 in 20,000 deliveries. The diagnosis is usually fortuitous in that the patient undergoes laparotomy for an adnexal mass that is subsequently found to be malignant. In many instances, the close observation of the pregnant patient has led to the discovery of a lesion in the earlier stages. These include not only malignant germ cell tumors and sex cord–stromal cell cancers but also some epithelial malignancies. If an ovarian malignant neoplasm is found at the time of abdominal exploration, the surgeon’s first obligation is to properly stage the disease, as outlined in Chapter 9 . Although the gravid uterus hinders the surgeon’s ability to access the retroperitoneum, every effort should be made to remove the tumor intact. The contralateral ovary should be carefully inspected and biopsied if anything suspicious is detected. In the scenario of a clinical stage I ovarian carcinoma, unilateral adnexectomy, omentectomy, unilateral pelvic and aortocaval lymph node sampling, peritoneal biopsies, and four-quadrant washings can be safely carried out during pregnancy, with chemotherapy reserved for those patients who are upstaged on histopathologic analysis. The chemotherapy regimen is similar to what is used for advanced disease (e.g., a platinum compound and a taxane for the epithelial cancers); however, for patients with FIGO stage IA or IB, grade I non–clear cell tumors, no chemotherapy will be recommended.
Malignant germ cell tumors in pregnancy
Fortunately, ovarian germ cell neoplasms in pregnancy are usually benign. Dermoid cysts are by far the most common neoplastic cysts found in pregnancy; however, malignant ovarian germ cell tumors such as the dysgerminoma, embryonal carcinoma, immature teratoma, and yolk sac tumor (formerly called “endodermal sinus tumor”) have also been reported. Although a considerable number of these cancers present with early-stage disease (in both pregnant and nonpregnant patients), there are several reports of advanced cancers associated with pregnancy. Combination chemotherapy during pregnancy has been given without deleterious effects on the fetus.
The management of malignant ovarian germ cell tumors is predicated on the histologic identity of the tumor (see Fig. 12.7 ). Patients with clinical stage IA dysgerminoma and those with clinical stage IA–IB grade I immature teratoma require surgical staging to determine the need for adjuvant chemotherapy ( Fig. 12.9 ). All other histologic types require adjuvant chemotherapy, and therefore unilateral adnexectomy is all that is typically accomplished at the time of laparotomy, along with removal of all gross metastatic disease. Adjuvant chemotherapy for stage IC/grade 3 immature teratoma and stage IB–IC dysgerminoma is controversial with some data supporting close surveillance only.
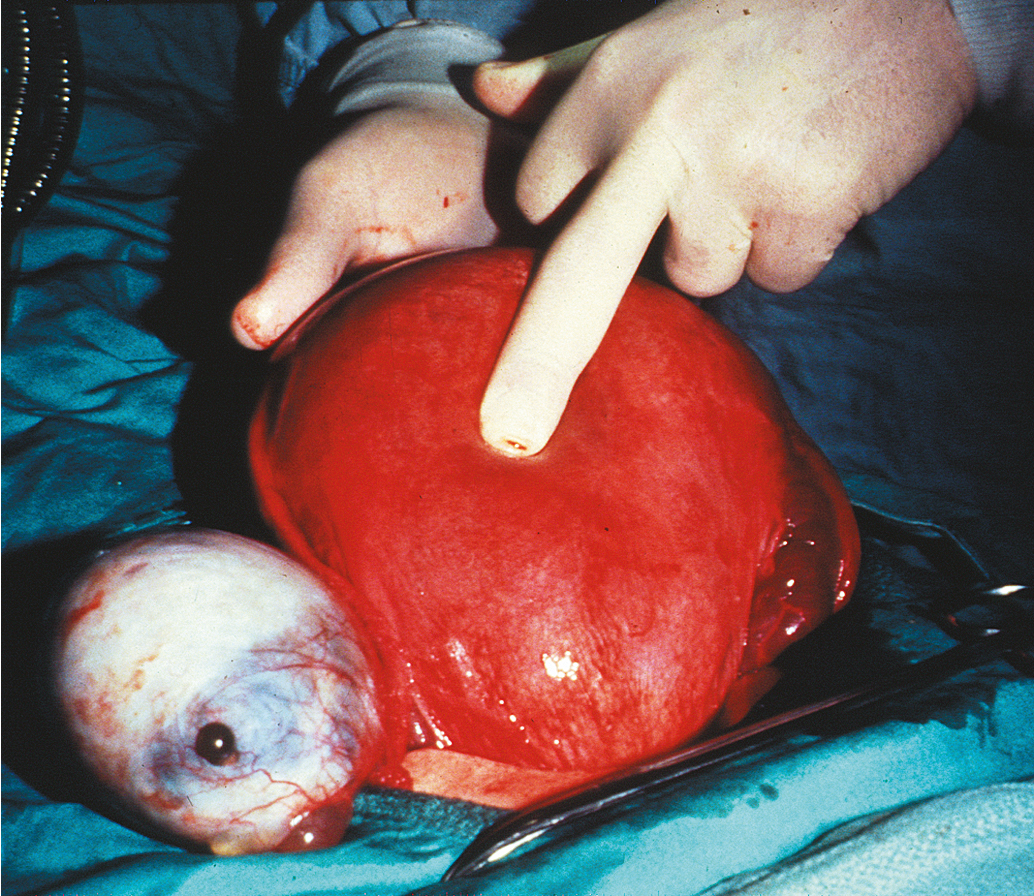
Because most malignant germ cell tumors are unilateral (the dysgerminoma is the exception in 10% of cases), it is inappropriate to remove both ovaries. Even when the opposite side may harbor an occult dysgerminoma, it is often not necessary to remove the entire contralateral ovary. If, however, both ovaries are grossly involved by malignancy and the pregnancy is in the second trimester and thus free from hormonal support by the corpus luteum, both ovaries should be extracted. The prognosis for this stage is not improved with more extensive surgery. Chemotherapy regimens currently used for this disease comprise bleomycin, etoposide, and cisplatin. This regimen has been used safely during pregnancy.
Combined chemotherapy has improved survival markedly for malignant germ cell ovarian tumors and can permit preservation of childbearing capacity and maintenance of the existing pregnancy if the disease is stage I. If the diagnosis is made during the first or second trimester, the patient must decide whether to permit the pregnancy to continue to viability before adjuvant chemotherapy is instituted. Because these tumors characteristically grow rapidly and often recur within months when therapy is withheld, delays in initiating systemic therapy can be harmful. Indeed, the high success rate obtained with adjuvant chemotherapy has been recorded with use of this modality in the immediate postoperative period. The effect of a treatment-free interval of several months before the commencement of adjuvant chemotherapy has not been tested adequately. Thus, a patient with a malignant ovarian germ cell tumor discovered early in pregnancy and in need of chemotherapy is faced with a dilemma for which no data are available. Malone and colleagues described a patient with stage IC endodermal sinus tumor diagnosed in the 25th week of gestation who was given two cycles of combination chemotherapy consisting of vinblastine, bleomycin, and cisplatin and delivered a healthy boy by cesarean section at 32 weeks of gestation. She subsequently completed three more cycles of chemotherapy and remained well at the time of Malone and colleagues’ report 18 months after initial diagnosis. To our knowledge, this was the first report of a case of a patient who had endodermal sinus tumor treated with combination chemotherapy during pregnancy that apparently had a successful outcome for both mother and infant. Subsequently, we and others have had similar experiences.
Therapeutic decisions for patients who have more advanced stages of these tumors are also difficult and controversial. Many such patients can be cured with early adjuvant chemotherapy after surgery. As in earlier stages, the uterus and opposite ovary can be preserved if metastatic tumor is not found in these locations. Some clinicians preserve the uterus and opposite ovary under all conditions in the hope that postoperative chemotherapy will sterilize those organs also. No long-term follow-up of this approach is available. Delays in withholding chemotherapy are not warranted, and uterine evacuation is often requested because of fear of potential teratogenic effects when chemotherapy is required during the first trimester. The subject of adjuvant chemotherapy in pregnancy is discussed later, but we emphasize that all chemotherapeutic agents are theoretically teratogenic. Although retrospective studies have not shown frequent congenital abnormalities in patients treated in the second and third trimesters, many newer agents have not been used frequently in pregnancy.
Dysgerminoma in pregnancy
Ovarian dysgerminomas are unique among the malignant germ cell tumors because of their overall good prognosis in FIGO stage IA treated by surgery alone. Dysgerminoma is particularly common and accounts for 30% of ovarian malignant neoplasms in pregnancy. We believe that these tumors can be managed with a unilateral adnexectomy and continuation of the pregnancy without additional therapy in FIGO stage IA. Optimal staging should include a pelvic and periaortic lymphadenectomy on the side of the tumor mass because dysgerminomas metastasize primarily through the lymphatic system to the ipsilateral pelvic and periaortic lymph nodes. Because lymphangiography and computed tomography (CT) are contraindicated when the pregnancy is to be continued, patients who have not had adequate exploration at initial surgery should be considered for reexploration before further therapy and continuation of the pregnancy are recommended. Appropriate diagnostic studies, including lymphangiography and CT scan of the abdomen and pelvis, may be performed in the postpartum period. A mass on scan or a suspicious lymph node on lymphangiography should be evaluated at reexploration.
Emergency surgical intervention and obstetric complications are common in patients with dysgerminomas. Karlen and associates reviewed 27 cases of dysgerminoma associated with pregnancy. Torsion and incarceration were found commonly in this group of patients who had rapidly enlarging neoplasms averaging 25 cm in diameter. Obstetric complications occurred in nearly half the patients, and fetal demise occurred in one-quarter of the reviewed cases. There were recurrences in 30% of 23 stage IA tumors treated by unilateral oophorectomy, which calls into question the philosophy of treating these patients conservatively. The extent of exploration was not known in most cases, however, and therefore accuracy of staging cannot be assessed. This information is essential for appropriate interpretation of the findings. In our experience, lesions that are confined to one ovary have a 10% recurrence rate. Although most of these lesions recur in the first 2 years after surgery, we believe that this group of patients can continue their pregnancies safely with completion of their proper evaluation in the puerperium. Because radiation therapy and chemotherapy are successful in curing more than 75% of patients, even those with metastatic or recurrent dysgerminoma, and because there is a low incidence of recurrence in patients with FIGO stage IA disease, we maintain a philosophy of conservatism for the treatment of these tumors.
Sex cord–stromal tumors in pregnancy
Granulosa and Sertoli–Leydig cell tumors together account for only 2% to 3% of all ovarian neoplasms. Although granulosa cell tumors are most commonly discovered in perimenopausal or postmenopausal women, 10% to 20% are encountered during the reproductive years. Sertoli–Leydig cell tumors occur in women in the reproductive age group in 74% of cases. Of note, sex cord–stromal tumors are rarely found in pregnancy. It is critical that these entities are distinguished from ovarian decidualization, luteoma of pregnancy, or even benign granulosa cell proliferations observed with pregnancy. Typically, the sex cord–stromal ovarian tumors behave as they do in nonpregnant women, presenting with early-stage disease and having a slow, low-grade, and indolent course. Thus, because their biologic behavior is akin to that of neoplasms of low malignant potential, it is recommended that they be managed conservatively (i.e., unilateral adnexectomy without comprehensive surgical staging) as in young nonpregnant patients (see Fig. 12.7 ). It is important, however, to resect all visible tumor whenever possible.
Young and colleagues reported a series of 17 granulosa cell, 13 Sertoli–Leydig cell, and 6 unclassified sex cord–stromal tumors diagnosed during pregnancy or the puerperium. Eleven patients had abdominal pain or swelling when they were first seen by a physician, five were in shock, two had virilization, and one had vaginal bleeding. Three asymptomatic patients underwent exploration because of palpable masses, and one underwent exploration because of an adnexal mass found on ultrasound examination. In 13 patients, the tumors were discovered during cesarean sections; 5 patients had dystocia, and the tumors were incidental findings in 8 patients. All of the tumors were FIGO stage I, but 13 of the tumors had ruptured. All but one were unilateral. Hemoperitoneum was present in seven cases.
Young and colleagues uncovered four major sources of difficulty in the interpretation of their series of 36 cases:
- 1.
The young age of the patients
- 2.
An alteration in the histologic appearance of the tumors during pregnancy
- 3.
A decreased frequency of associated endocrine manifestations
- 4.
Pregnancy-induced changes in other neoplastic and nonneoplastic lesions of the ovary that cause them to simulate sex cord–stromal tumors both morphologically and in terms of endocrine function.
Indeed, the pregnancy-associated tumors commonly exhibited alterations related to the pregnant state that tended to obscure characteristics and familiar features that are apparent in tumors removed from nonpregnant patients. The most striking changes included intercellular edema and increased extent of luteinization in the granulosa cell tumors and of Leydig cell maturation in the Sertoli–Leydig cell tumors. It is important to note that the edema often blurred the architectural patterns of the tumors and distorted the cytologic features of the neoplastic cells. The marked luteinization and Leydig cell maturation interfered with the recognition of these cells. The end result was that these pregnancy-associated changes made identification of the tumor type more difficult.
Granulosa cell tumors are clinically estrogenic in approximately two-thirds of cases, and Sertoli–Leydig cell tumors are virilizing in nearly 50% of cases. These hormonal manifestations often suggest the correct diagnosis to the gynecologist. However, during pregnancy, the hyperestrogenic clinical manifestations do not appear, with none of the 17 tumors in the series by Young and colleagues associated with estrogenic manifestations. It is quite probable, however, that many of these tumors were secreting estrogens in large quantities. Furthermore, only 16% ( n = 5) of the 36 sex cord–stromal tumors were associated with clinical evidence of excess androgens (virilization in four cases and hirsutism in only one case). This low frequency may have been the result of the placenta’s ability to aromatize androgens produced by the tumor. In fact, one of the masculinizing tumors was the largest in their series (32 cm in maximal diameter) and may have been secreting androgens in such great abundance that the aromatizing capacity of the placenta was exceeded.
Several other types of ovarian neoplasms and nonneoplastic disorders are more frequently associated with virilization during pregnancy than Sertoli–Leydig cell tumors. Tumors with functioning, proliferative ovarian stroma such as Krukenberg tumors and the mucinous cystic tumors may be confused morphologically with sex cord–stromal tumors. Other primary ovarian tumors that can cause virilization include luteinized thecomas and Leydig and lipid cell tumors. Finally, as described earlier, two nonneoplastic lesions of the ovary that develop during pregnancy and can be associated with virilization include the luteoma of pregnancy and the hyperreactio luteinalis (multiple luteinized follicle cysts). All of these virilizing tumors and lesions must be considered in the differential diagnosis when confronted with virilization of the pregnant mother so as to not make an erroneous clinical diagnosis of a sex cord–stromal tumor.
With one exception, the patients in the study by Young and colleagues were initially treated by conservative surgical procedures. Two of them received chemotherapy, and two received radiation therapy postoperatively. Hysterectomies and salpingo-oophorectomies were performed as second operations in eight cases; no residual tumor was found in any of these specimens. Only one patient had a recurrence, which was treated surgically. Follow-up for the average of 4.7 years was available for 30 of the 36 patients; all of them were free of disease at their last examination. Adjuvant therapy has not been demonstrated to improve the outcome in this group of patients and is not recommended during pregnancy.
Chemotherapy for nonepithelial ovarian cancer during pregnancy
A recent literature review by Azim and colleagues identified 18 cases of nonepithelial ovarian malignancies treated with systemic chemotherapy during pregnancy. Most patients had a yolk sac tumor ( n = 10) and were treated after the first trimester with the bleomycin, etoposide, and cisplatin (BEP) regimen or the cisplatin, vinblastine, and bleomycin (PVB) regimen. Other histologies reported included three cases of dysgerminoma, three cases of immature teratoma, one mixed germ cell tumor, and one Sertoli–Leydig tumor. Fifteen of the 18 women completed their pregnancies without complications; there was one case of premature delivery after maternal dehydration at 28 weeks after BEP, there was one case of pregnancy-induced hypertension after three cycles of BEP, and a third patient developed oligohydramnios after three cycles of etoposide-platinum. Postnatal chemotherapy-induced adverse events were restricted to one baby who developed minor anemia and transient respiratory distress at delivery at the 35th week of gestation. This baby had been exposed to three cycles of carboplatin plus paclitaxel for a maternal dysgerminoma, and the condition resolved quickly, and the authors reported normal development at 20 months of follow-up.
Epithelial ovarian cancer in pregnancy
Very few series of malignant ovarian carcinomas in pregnancy have been published. A recent series of 105 cases of epithelial ovarian cancer found serous carcinomas comprised the largest histologic subtype (47.6%) followed by mucinous (27.6%) and endometrioid (10.5%). Nearly half were diagnosed during the first trimester (45.3%), and there were 78 live births (81.3%), 41 of which were at term (57.7%). Surgery was performed predominantly during the second trimester (43.0%), with unilateral adnexectomy alone most commonly performed (63.4%). Hysterectomy was performed during pregnancy in 16 (15.8%) and omentectomy in 21 (20.8%). The majority of cases were FIGO stage I at diagnosis (63.8%) followed by stage III (24.9%). It is quite certain that antenatal care, including serial physical examinations and ultrasonography, contributed to these early-stage diagnoses.
After the diagnosis of ovarian carcinoma is made during pregnancy, appropriate therapy should not be withheld (see Fig. 12.7 ). In patients who present with metastatic disease manifesting as malignant ascites and carcinomatosis, surgical exploration is warranted, with an attempt to remove as much of the tumor burden as is feasible. Depending on the degree of tumor involvement of the uterus and the mother’s desire for the pregnancy, uterine preservation may be considered, and if so, the “hands-off” approach to the uterus and removal of mobile intraperitoneal deposits can be attempted. Certainly, the typical aggressive cytoreductive approach (e.g., bowel resection, splenectomy) taken in nonpregnant women with advanced-stage ovarian carcinoma can result in significant morbidity, and if the pregnancy is to be continued, we advise against this approach unless the patient has presented with a bowel obstruction.
Recently, multiple phase 3, randomized control trials have been published demonstrating the non-inferiority of a neoadjuvant chemotherapy approach when compared to primary debulking surgery for primary treatment of epithelial ovarian cancer. The Society of Gynecologic Oncology (SGO) and the American Society of Clinical Oncology (ASCO) have provided guidelines for the use of neoadjuvant chemotherapy for advanced ovarian cancer. While explicit recommendations for pregnant women are not addressed, neoadjuvant chemotherapy is recommended in patients with high perioperative risk and/or low likelihood of achieving cytoreduction to less than 1 cm of residual disease. Accordingly, neoadjuvant chemotherapy can be considered in pregnant women during the second and third trimester.
The standard regimen for metastatic epithelial ovarian cancer in nonpregnant patients includes platinum-based chemotherapy. Regimens containing cisplatin alone and cisplatin plus paclitaxel and even administration of a full six cycles of carboplatin and paclitaxel (the current standard among nonpregnant women) have been used during the second and third trimesters of pregnancy. Because there does not appear to be any significant risk to the fetus when these drugs are used in the second and third trimesters, pregnant patients diagnosed during these periods should be offered the opportunity to receive platinum-based therapy without terminating their pregnancies. Postpartum, the patient may return to the operating room to undergo definitive surgical staging or comprehensive tumor debulking.
Patients remote from term (e.g., during the first trimester) with metastatic disease should be advised to undergo hysterectomy with the fetus in situ in conjunction with tumor debulking. Because the prognosis for women with advanced carcinoma is poor, patients must be counseled regarding the realities of how much time they would have with their child when making decisions regarding pregnancy termination.
It should be noted that there are at least two reports of an advanced-stage epithelial carcinoma of the ovary in which the uterus and pregnancy were preserved at 15 and 20 weeks of gestation. Patients were treated with six cycles of single-agent cisplatin or four courses of carboplatin plus paclitaxel followed by cesarean section and completion hysterectomy. One patient was disease free at a short follow-up of 6 months, but the other recurred in the pelvis at 24 months after delivery. This second patient underwent secondary cytoreduction and was retreated with platinum-based therapy and has remained disease free at 42 months. Although some have advocated sparing the uterus if it appears to be uninvolved and the pregnancy is remote from term, this approach should be used with extreme caution when dealing with advanced-stage epithelial ovarian cancer for which the outcome remains exceedingly poor irrespective of pregnancy status.
Chemotherapy for epithelial ovarian cancer during pregnancy
Azim and colleagues have collected 20 patients from the literature who were treated with chemotherapy during pregnancy for epithelial ovarian cancer. The histologic subtypes included serous ( n = 13), mucinous ( n = 3), endometrioid ( n = 2), clear cell ( n = 1), and undifferentiated ( n = 1). The chemotherapy regimens used included cyclophosphamide plus cisplatin ( n = 5); single-agent cisplatin ( n = 4); single-agent carboplatin ( n = 3); paclitaxel plus carboplatin ( n = 2); and cyclophosphamide, doxorubicin, and cisplatin ( n = 2). There also was one case each of the use of the following regimens: cisplatin plus paclitaxel, cisplatin plus docetaxel, carboplatin plus cyclophosphamide, and single-agent paclitaxel. Seventeen of the patients received their first cycle during the second trimester and three in the third trimester. Sixteen patients delivered at week 34 or after, and 16 had experienced no pregnancy-related complications. There was one case each of intrauterine growth restriction (IUGR), premature rupture of the membranes, and preeclampsia. Nineteen fetuses had a normal outcome, with nine of these children reported to be normal at 1 year or longer follow-up. There was one neonatal death from multiorgan failure in a baby with congenital anomalies identified before starting cisplatin plus docetaxel at week 20.
Intravenous carboplatin plus paclitaxel is the most commonly used regimen for epithelial ovarian cancer. Although use of platinum derivatives appears to be feasible during the second and third trimesters, their administration does raise concern regarding the transplacental transfer of these drugs in late pregnancy, and the short- and long-term effects have not been well-studied. Intraperitoneal chemotherapy in combination with intravenous chemotherapy has been associated with higher survival rates for patients with epithelial ovarian cancer. In patients opting for this type of therapy, delivery should be induced as soon as fetal pulmonary maturation can be documented before starting intraperitoneal therapy. Catheters can be placed at the time of cesarean section or laparoscopically in the postpartum period if the pregnancy was delivered vaginally.
Targeted (biologic) therapy for ovarian cancer in pregnancy
Vascular endothelial growth factor inhibitors.
After an 8-year drought beginning in 2006, during which time there were no new drug approvals for ovarian cancer (in nonpregnant patients), finally in 2014, the management of epithelial ovarian cancer entered the modern area of targeted therapy with two approvals by the US Food and Drug Administration (FDA). Bevacizumab, a fully humanized monoclonal antibody (mAb) that targets the vascular endothelial growth factor (VEGF) ligand and sequesters it to prevent binding to the VEGF receptor (VEGFR) and thereby prevent tumor angiogenesis was approved in combination with chemotherapy (physician’s choice of pegylated liposomal doxorubicin or topotecan or weekly, dose-dense paclitaxel) for platinum-resistant recurrent ovarian carcinoma. Since then, bevacizumab has been approved by the FDA for maintenance in newly diagnosed ovarian cancer that has responded to primary chemotherapy with or without bevacizumab. Importantly, the combination of chemotherapy plus bevacizumab was approved in 2014 for recurrent/metastatic/persistent cervical carcinoma.
It is unclear whether mAbs are safe when used to treat cancer in pregnancy. Sarno et al. performed a literature search encompassing the years 2000 through January 2013 and reviewed the safety of mAbs during pregnancy. As will be discussed later under the section concerning breast cancer in pregnancy, the anti-HER2 mAb, trastuzumab, has been associated with oligohydramnios and poor neonatal outcomes. There are very few reports on the use of bevacizumab in pregnant women, but because its antiangiogenic effects may have possible negative consequences on fetal development, the consensus that has been reached is one in which this drug should be avoided during pregnancy. Not only has VEGF blockade during pregnancy been associated with maternal preeclampsia and fetal IUGR, but in murine models, administration of anti-VEGF receptor tyrosine kinase inhibitors (TKIs) has led to abnormal vascular patterning in the mouse retina. Another VEGF receptor TKI, sunitinib, has been associated with embryo and fetal developmental toxicity in rats and rabbits at clinically relevant dose levels. Parenthetically, there have been reports of intravitreal bevacizumab being given safely to pregnant women for choroidal neovascularization with resulting improvement in vision and no untoward fetal effects. However, given the significant potential for adverse fetal developmental outcome resulting from inhibition of the VEGF access, the use of mABs and oral TKIs in pregnancy should be avoided.
PARP inhibitors.
Poly (ADP-ribose) polymerase inhibitors (PARPi) are small molecule inhibitors that trap PARP1 proteins which are important for the repair of single-strand DNA breaks. By inhibiting PARP-dependent DNA repair, PARPi(s) are particularly effective in patients with homologous recombination deficiencies, such as patients with BRCA mutations. PARPi(s) have demonstrated clinical benefit for use in up-front maintenance of ovarian cancer, as well as in the treatment and maintenance in platinum-sensitive recurrent ovarian cancer. Between 2014 and 2020, the FDA has approved three different PARPi(s) for use in ovarian cancer—olaparib, niraparib, and rucaparib. In addition, PARPi(s) are approved by the FDA for treatment of germline BRCA-deficient, HER-2 negative breast cancer (talazoparib, olaparib), as well as homologous recombination deficient metastatic castration-resistant prostate cancer (olaparib), and germline BRCA-deficient metastatic pancreatic cancer (olaparib, rucaparib). To date, there is no data regarding the use of PARP inhibitors in human pregnancy. In murine models, PARP1 upregulation is crucial for embryo implantation. Interestingly, PARP inhibition has been shown to prevent the development of endothelial dysfunction and hypertension in rat models of preeclampsia. Nevertheless, due to the lack of data, PARP inhibitors should be avoided in pregnancy.
Other types of malignant ovarian tumors
Some ovarian cancers are particularly aggressive, and chemotherapy should be started immediately upon diagnosis. These include small cell ovarian cancer and metastatic Krukenberg tumors. The only long-term survivor of an advanced-stage small cell ovarian cancer was reported by Tewari and colleagues, who described a patient diagnosed with the disease during pregnancy. After the histology was confirmed, the patient returned to the operating room in the second trimester and underwent cytoreductive surgery with the fetus in situ and postoperatively was treated with a six-drug regimen that contained both epithelial-cell and germ-cell activity. Another patient diagnosed with small cell ovarian cancer with hypercalcemia underwent conservative surgery during pregnancy followed by chemotherapy and died of disease 10 months after diagnosis. Taylor and colleagues reported the surgical removal of a Krukenberg tumor at 15 weeks of gestation followed by treatment with 10 cycles of 5-fluorouracil (5-FU), folinic acid, and irinotecan every 2 weeks until the 36th week of pregnancy. The neonate was born without complications and at age 4 months showed normal development.
Summary of the adnexal mass and ovarian cancer in pregnancy
The problem of an adnexal mass in pregnancy is simple: one must have a high index of suspicion for malignancy, make the diagnosis early, and treat promptly. The difficulty arises when both patient and physician resist abdominal exploration during pregnancy because of fear of precipitating fetal wastage. However, the potential danger to the mother far exceeds the imagined danger to the child. Most of the difficulties seen with ovarian tumors are those of omission rather than of commission. The probability of ovarian cancer must be kept foremost in the minds of physicians caring for these patients. At laparotomy, most malignant ovarian tumors apparently confined to one ovary will require complete surgical staging. A technique of “hands-off-the-uterus,” whenever possible, appears to reduce postoperative uterine contractions.
Breast cancer
Pregnancy-associated breast cancer (PABC) is defined as breast cancer diagnosed during pregnancy or lactation up to 12 months postpartum. Because breast cancer is rare in women younger than 35 years, this problem, fortunately, is a rare complication of pregnancy, with an incidence of approximately one case for every 3000 deliveries. Conversely, of all patients with breast cancer, 1% to 2% are pregnant at the time of diagnosis. PABC provides a challenging scenario for the mother and oncologist. It is the most common malignancy to complicate pregnancy, but unlike cervical cancer, it is not screened for during pregnancy, and because delays in diagnosis are common and the diagnosis itself is elusive, often patients are diagnosed with advanced tumors for which prognosis is poor. Furthermore, the management of breast cancer often involves a coordination of surgery, radiotherapy, chemotherapy, and even hormonal therapy, all of which may affect the pregnancy. Finally, there are several distinct hormonal issues related to pregnancy that may have an influence on the course of breast cancer.
Recently, a group of specialists convened to review guidelines and provide guidance on how to implement recent advances in breast cancer diagnosis and treatment in the pregnant population. The consensus was that the majority of women with PABC will be considered for definitive treatment during pregnancy and that many treatments, including sentinel lymph node biopsy (SLNB) and systemic therapy with taxanes, platinum agents, and dose-dense regimens can be given safely after the first trimester of pregnancy. Radiation therapy and anti-HER2–directed therapy (e.g., trastuzumab, lapatinib, trastuzumab emtansine [T-DM1], and pertuzumab) are best avoided during pregnancy but may be considered in some rare instances.
Prognosis
Historically, PABC has been associated with a poor prognosis, with accounts from the 19th century describing exceedingly rapid growth and a malignant course. In 1943, Haagensen and Stout reinforced this feeling of doom when they decided that the outcome for this group of patients was so poor that they recommended surgical treatment not be offered. Contemporary opinion for the most part maintains the dismal prognosis associated with PABC. It must be acknowledged that the literature comprises mainly single-institution retrospective experiences and case reports. The only series containing greater than 100 patients are 4 in number (White, Bunker and Peters, Ribeiro and Palmer, Clark and Reid), none of which were published after 1978.
Although the overall survival rate for breast cancer is greater than 60%, the overall survival rate in pregnancy is reported by some to have dropped to 15% or 20%. Pregnant patients tend to have a higher incidence of positive axillary lymph nodes. Locoregional spread of the tumor portends a poor prognosis and in all likelihood suggests that the neoplasm has metastasized at the time of the initiation of therapy. The advanced stage of the presentation of disease in pregnant patients has been attributed to multiple factors. First, the engorged breast can successfully obscure a lesion for a much longer period. Survival rates are lower for cases diagnosed late in pregnancy than for those recognized in the first trimester. Others emphasize the 30 to 50 multiples of increase in serum levels of estrogens and progesterone. In addition, there may be increased vascularity and lymphatic drainage from the pregnant breast, assisting the metastatic process to regional lymph nodes. If a lesion is detected early (present <3 months, smaller than 2 cm, and no positive nodes), the chance of survival (70% to 80%) is the same for pregnant and nonpregnant patients. If, however, there is involvement of the subareolar region; diffuse inflammatory carcinoma; edema or ulceration of the skin; fixation of the tumor to the breast wall; or involvement of the high axillary, supraclavicular, or internal mammary nodes, the prognosis is poor for both pregnant and nonpregnant patients.
Presentation
At least 10% of patients with breast cancer who are younger than 40 years will be pregnant at diagnosis. PABC typically presents as a painless mass or thickening. In some cases, there may be an associated nipple discharge, and in the lactating breast, the infant may exhibit the “milk rejection sign,” effectively refusing the breast that contains the cancer. The mean breast weight normally doubles in pregnancy from 200 to 400 g, resulting in breast firmness and increased breast density. Mammographic evaluation of the pregnant breast is difficult to interpret, and the clinical examination may be deceptive. This has profound implications in terms of delays in diagnosis and treatment, which, as discussed earlier, are common in PABC. Many patients with PABC will have a delay in diagnosis ranging from 1 to 2.5 months during pregnancy and up to 6 months during lactation. In a 1991 series from the Memorial Sloan-Kettering Cancer Center in New York, 44 of 56 patients did not have the diagnosis of cancer made until the postpartum period. Overall, in a review of the literature by Puckridge and colleagues, a delay of 2 to 15 months longer from manifestation of the first symptoms to the diagnosis of cancer occurs in PABC. Given the tumor-doubling time of 130 days, a 1-month delay in primary tumor treatment increases the risk of axillary metastases by 0.9%, and a 6-month delay increases the risk by 5.1%. For this reason particularly, PABC has been considered an ominous diagnosis, but when age and stage are taken into account, there is no difference in the survival rate of PABC cases compared with non-PABC cases. Pregnancy is not thought to be an independent risk factor.
Evaluation
A proposed management algorithm for PABC appears in Fig. 12.10 . Early diagnosis has been associated with improved survival rates and relies on the liberal use of imaging strategies and the core and fine-needle biopsy techniques for this group of patients. Mammography in conjunction with abdominal lead shielding can be safely used during pregnancy, but as discussed earlier, the engorged and lactating breast increases tissue density and may mask abnormalities. Ultrasonography yields equivalent information with no known adverse effects to the fetus. Fine-needle aspiration (FNA) may be difficult to interpret cytologically secondary to cellular changes that take place during pregnancy and lactation and is often associated with an increase in the false-negative rate. Core biopsy remains the gold standard in making the diagnosis. When necessary, an open biopsy under local anesthesia is also appropriate. Stopping lactation with ice packs and breast binding or bromocriptine (2.5 mg three times daily for 1 week) beforehand will reduce the risk of a milk fistula. The breasts should be emptied of milk before the biopsy, and a pressure dressing will decrease the risk of hematoma that may develop from the hypervascularity of the pregnant breast.
Approximately 75% to 90% of PABCs are ductal carcinomas, mirroring what is observed in the nonpregnant population. Historically, there was a perceived increase in inflammatory carcinoma of the breast during pregnancy; however, this has since been refuted in contemporary series, in which the incidence ranges from 1.5% to 4.2% among pregnant and nonpregnant patients. Several studies have demonstrated adverse pathologic features in PABC. Most patients with PABC have estrogen receptor (ER)–negative and progesterone receptor (PR)–negative tumors. This may be a result of the production of false-negative results by the ligand-binding assay used for ER and PR when high circulating levels of estrogen and progesterone downregulate receptors. Immunohistochemistry has not been able to detect a difference in the number of hormone receptor–positive tumors when PABC cases are compared with cases of breast cancer in nonpregnant patients of similar ages. Additionally, higher levels of c-ERBB-2 overexpression and p53 mutations have been reported in lactational carcinomas but not in tumors diagnosed during pregnancy. Furthermore, there have been reports of increased HER-2/neu–positive tumors compared with nonpregnant control participants. It is interesting that the HER-2/neu oncogene product p105 is overexpressed not only in ductal carcinomas but also in fetal epithelial cells and the placenta and that toward the end of the third trimester of pregnancy, serum levels of p105 normally rise.
It is known from epidemiologic studies that there is an increased incidence of breast cancers in certain families; the risk increases 5 to 10 times if a patient’s mother or sister has had the disease. It is interesting that women with a genetic predisposition to breast cancer may be overrepresented among cases of PABC, with a significant family history of breast cancer being three times more common in women with PABC than among nonpregnant patients with breast cancer. Along these lines, PABC has been associated with a higher rate of BRCA2 allelic mutation compared with sporadic breast cancer. Indeed, a Swedish report of 292 women with breast cancer before the age of 40 years demonstrated a greater likelihood of known BRCA1 and BRCA2 carriers to develop cancer during pregnancy.
Staging of breast cancer currently uses a complicated system jointly recommended by the International Union Against Cancer and the American Joint Committee on Cancer (AJCC) (see Table 11.11 ). The Haagensen clinical staging for breast cancer is more useful in pointing out the unfavorable prognostic indicators in this disease process. Lateral and posteroanterior chest radiographs in conjunction with lead shielding are considered safe during pregnancy, with an estimated fetal dose of only 0.6 mGy. Provided a catheter is placed to allow rapid drainage of radioactive material from the bladder, a low-dose labeled technetium-99 bone scan is also safe. The low-dose bone scan exposes the fetus to 0.0008 Gy instead of the standard 0.0019 Gy. The higher radiation exposure to the fetus excludes the use of CT in planning a metastatic workup, but MRI may be used to study the thorax and abdomen and to image the skeleton. MRI is preferred to ultrasonography for hepatic imaging and is also the safest and most sensitive way to study the brain.
Surgical management
Surgery is the definitive treatment for PABC (see Fig. 12.10 ). The extent of surgery in the treatment of breast cancer is being debated throughout the world, and this issue cannot be adequately addressed here. Lumpectomy or partial mastectomy is more commonly used, especially when the lesion is not large, although the preferred surgical treatment for stage I, stage II, and some stage III tumors involves mastectomy, thus avoiding the need for adjuvant radiotherapy in most cases (i.e., early-stage breast cancer). Because nodal metastases are commonly identified in PABC and nodal status dictates the choice of adjuvant chemotherapy, axillary dissection has been routinely recommended, especially in light of the potential risk to the fetus from radioisotope if SLNB was attempted.
The timing of surgery for cancer diagnosed late in pregnancy is another source of debate. Some reports suggest that patients treated postpartum survive longer than those treated in the second and third trimesters. This suggests that postponement of therapy for patients near term may be of benefit. These reports fail to consider the possibility that patients selected for postponed treatment might have been those with small, more favorable cancers discovered late in pregnancy, but larger, aggressive, anaplastic cancers with rapid progression received immediate treatment. If such treatment bias exists, prompt treatment would not be expected to correlate with good results, and treatment after delivery would appear favorable because of a preponderance of favorable patients in that group.
Sentinel lymph node identification
Keleher and colleagues assessed the risk to the embryo or fetus associated with SLNB and lymphoscintigraphy of the breast in pregnant patients. After peritumoral injection of 92.5 MBq (2.5 mCi) of filtered 99m Tc sulfur colloid the day before surgery in two nonpregnant women with breast cancer, they calculated the absorbed dose to the embryo or fetus for three theoretic extreme scenarios of biodistribution and pharmacokinetics and described the maximum absorbed dose to the fetus being 4.3 mGy calculated for the worst-case scenario. The authors concluded that breast lymphoscintigraphy during pregnancy presents a very low risk to the embryo or fetus. In 2008, Khera and colleagues searched a prospectively accrued breast database for cases of SLNB in patients with PABC and identified 10 patients. The mean gestational age at the time of biopsy was 15.8 weeks. All patients were successfully mapped, and a positive SLN was identified in five patients (50%). Nine patients (90%) delivered healthy babies without reported complications, and one patient elected to terminate the pregnancy in the first trimester to start chemotherapy. The authors concluded that SLNB can be safely performed in pregnancy with minimal risk to the fetus.
Spanheimer and colleagues measured abdominal, perineal, and urinary radiation in 14 women with breast cancer and total uterine doses were calculated. The average dose to the uterus from bladder radioactivity determined from voided urine was 0.44±0.44 microGy. The average radiation dose to the uterus of 1.14±0.76 microGy was derived through an average of abdominal and perineal doses plus contribution from the bladder dose. One patient was 16 weeks pregnant at the time of SLNB, and the total calculated uterine dose was 1.67 microGy, suggesting that pregnancy does not significantly alter measured uterine radiation. When compared with average background radiation (i.e., 3000 microGy/year or 8.2 microGy/day), it was concluded that the measured uterine dose of radiation from lymphoscintigraphy for SLN biopsy was significantly less than the average daily background radiation.
In a recent report by Gentilni and colleagues, 12 patients with PABC received low-dose (10 MBq on average) lymphoscintigraphy using 99m Tc human serum albumin nanocolloids. The SLN was identified in all patients. In 10 patients, the SLN was pathologically negative. One patient had a micrometastasis in an SLN, and another who had a metastasis in the SLN underwent axillary clearance. Eleven healthy babies were born with no malformations and appropriate birth weight. One baby who underwent lymphatic mapping during the 26th week of gestation was operated on at the age of 3 months for a ventricular septal defect and at 43 months was in good health. The malformation was suspected at the anatomy scan performed during the 21st week of gestation, well before lymphoscintigraphy. At a median follow-up period of 32 months, no overt axillary recurrence appeared in the patients with negative SLNs. This experience, together with those described earlier, supports the safety and efficacy of SLN identification in women with PABC when performed with a low-dose lymphoscintigraphic technique.
Breast reconstruction
Although the performance of a transverse myocutaneous flap of the rectus abdominis muscle (TRAM flap) is a satisfying, one-step, immediate reconstruction of good aesthetic quality without need for prosthetic materials, it is not recommended in patients with PABC who undergo mastectomy. The procedure should be deferred to the postpartum period. The problem of residual functional capacities of the abdominal wall deprived of a part or of all its rectus muscle also has important implications for future pregnancies. In fact, for some authors in the past, the desire for future childbearing was a contraindication to TRAM flap breast reconstruction because it was thought that the abdominal wall, weakened by rotation of one or both of its rectus muscles, would not be capable of withstanding the stress induced by pregnancy. To avoid the development of a hernia in the abdominal wall at the donor site, an interval of at least 12 months between breast reconstruction with a TRAM flap and pregnancy is recommended.
Adjuvant therapy
Women with early lesions may opt for a tissue-sparing procedure. In these cases, local irradiation is often required. Radiotherapy to the breast, chest wall, or axillary lymph nodes, even with shielding, results in a significant fetal dose because of scatter in excess of that which is considered safe. The doses of internal scatter of radiation have been calculated. Embryonic exposure resulting from breast radiotherapy with a dose of 0.1 Gy during organogenesis in the first trimester not only increases the risk of malformations but can also cause mental retardation. Using anthropomorphic phantoms simulating the geometry of a pregnant woman, the dose to the fetus resulting from tangential breast irradiation in each trimester has been well-summarized by Toesca et al. in their review of locoregional treatment of PABC. With fetal shielding and x-ray energies from 4 to 10 MV as used for breast radiotherapy, the fetal irradiation dose in the first and second trimesters is lower than the threshold values associated with organ malformations. However, during the third trimester when the fetus moves out of the pelvis and into the abdomen, the dose exceeds this threshold. For example, at 12 weeks of gestation, the fetus would receive 10 to 15 cGy. In the third trimester, this dose can be as high as 200 cGy. This is because, although during the first trimester the fetus is in a safer position and can be better shielded, it is more sensitive to the effects of radiation. In contrast, as the fetus enlarges and is less sensitive to radiation, it moves upward out of the pelvis, where it is less readily shielded and exposed to higher levels of ionizing radiation. Local irradiation should be deferred until after delivery of the fetus (see Fig. 12.10 ). Women treated with adjuvant radiation during the postpartum period will not be able to lactate from the irradiated breast.
Locally advanced disease is difficult to manage with the pregnancy in place. Chemotherapy or local radiotherapy, followed in 6 weeks by mastectomy, is the usual treatment plan for these lesions. For advanced disease, chemotherapy has been used after the first trimester when the mother is reluctant to terminate the pregnancy and the disease appears to be progressing at an alarming rate. The issue of whether chemotherapy should be administered to patients with node-positive breast cancer in pregnancy is complicated by reports suggesting that both single-agent and combination chemotherapy may significantly improve survival rates in premenopausal patients in an adjuvant setting. Follow-ups of 10 and 15 years are always necessary in breast cancer, but it appears that premenopausal patients are the best candidates for aggressive adjuvant chemotherapy and a resulting improved survival rate. This is especially pertinent to patients in whom positive nodes are discovered at the time of the initial procedure.
Table 12.8 contains reports of chemotherapy use during pregnancy, with favorable neonatal outcomes noted when treatment was administered only after the first trimester.
| Authors | Year | Patients ( n ) | Treatment | Trimester | Outcome |
|---|---|---|---|---|---|
| Tobias and Bloom | 1980 | 1 | AV, prednisone | 2 | No anomaly |
| Murray et al. | 1984 | 1 | AC, radiation | 1 | Imperforate anus, rectovaginal fistula |
| Mulvihill et al. | 1987 | 1 | CMF, melphalan | n/s | Spontaneous abortion |
| Zemlickis et al. | 1992 | 2 | CMF, melphalan (both cases) | 1 | Spontaneous abortion |
| 1 | CAFV, tamoxifen | 1 | No anomaly | ||
| 1 | CAF, tamoxifen | 3 | No anomaly | ||
| 1 | CMF | 3 | No anomaly | ||
| Cullins et al. | 1980 | 1 | Tamoxifen | 1, 2, 3 | Goldenhar syndrome |
| Tewari et al. | 1997 | 1 | Tamoxifen | 1, 2 | Ambiguous genitalia |
| Turchi and Villasis | 1988 | 1 | CAMF | 1 | No anomaly |
| Berry et al. | 1999 | 24 | CAF | 2, 3 | No anomaly |
| Isaacs et al. | 2001 | 1 | Tamoxifen, 0.0017 Gy RT | 1, 2, 3 | Preauricular skin tags |
| Andreadis et al. | 2004 | 1 | FEC, a 28 Gy RT, b tamoxifen, c zoledronic acid c | 1, 2, 3 | No anomalies |
| Gonzalez-Angulo et al. | 2004 | 1 | Neoadjuvant paclitaxel | 2, 3 | No anomalies |
| Warraich and Smith | 2009 | 1 | T, tamoxifen | 1, 2, 3 | Pulmonary hypoplasia |
| Baele et al. | 2009 | 1 | T, tamoxifen | 1, 2 | Respiratory failure |
| Azim et al. | 2009 | 1 | T | Preconception | No anomaly |
In a recent review by Azim and colleagues, the authors identified 56 different reports that have described the systemic treatment of PABC and separated them into four groups, as described in the following discussion.
Neoadjuvant and adjuvant chemotherapy for pregnancy-associated breast cancer
The FAC (5-FU, doxorubicin, and cyclophosphamide) regimen is the most commonly administered regimen in the neoadjuvant or adjuvant setting. The safety of this regimen was prospectively examined by Hahn and colleagues at the MD Anderson Hospital and Tumor Institute in Houston. Fifty-seven patients were treated, and the regimen was well tolerated and did not adversely affect the pregnancy. All women had live births, with three congenital defects reported, including Down syndrome, clubfoot, and congenital bilateral ureteral reflux. The investigators did not consider these anomalies to have resulted directly from chemotherapy exposure when the incidences were compared with those in the general population. Eighteen of the children have been followed until school age, and only two required special attention schools (including the child with Down syndrome).
Peccatori and colleagues from the European Institute of Oncology in Milan prospectively evaluated the safety of weekly epirubicin (35 mg/m 2 ) starting in the second trimester in 20 women with PABC. The schedule was selected to potentially allow lower peak plasma concentration of the drug, thus lowering the risk of maternal myelotoxicity and possible placental transfer of the drug. There were no grade III or IV toxicities and only one congenital anomaly (polycystic kidney). All children have had normal development at 2 years median follow-up.
Based on these two studies and other retrospective series and case reports from the literature, Azim and colleagues suggested that anthracycline-based regimens are probably safe starting in the second trimester. FAC is the most commonly studied regimen, but offering epirubicin instead of doxorubicin is an option.
In recent years, taxanes have been incorporated into the adjuvant and neoadjuvant settings to improve outcomes. There have been seven reports of docetaxel treatment and two reports of paclitaxel therapy for PABC. In six of these cases, the taxane was given as a single agent. None of the nine cases were associated with a poor obstetric or fetal outcome. Although the experience using taxanes in PABC is clearly limited, this early evidence is reassuring.
The CMF (cyclophosphamide, methotrexate, 5-FU) regimen has been used for many years as standard adjuvant therapy in breast cancer. As a result of the potential for teratogenicity by methotrexate (after first-trimester exposure), this regimen is not recommended for PABC.
Chemotherapy for metastatic breast cancer
In contrast to early PABC for which neoadjuvant or adjuvant therapy is administered in a good-prognosis setting, patients with metastatic disease have fewer options because treatment of the mother must take priority, especially in pregnancies remote from term. Anthracyclines remain the most studied agents for management of metastatic breast cancer, but data are extremely limited in PABC. Patients with bone metastases are frequently treated with bisphosphonates to decrease the risk of skeletal events. There has been some concern for the development of genital and skeletal defects with bisphosphonate use in pregnancy. In addition, the potential for hypocalcemia exists with bisphosphonate therapy, which may lead to stimulation of uterine contractions. There has been very limited experience in PABC, but no adverse events associated with bisphosphonate use have been reported. Among 21 pregnant women exposed to bisphosphonates for osteoporosis during the first trimester, there were no adverse events for the fetus or to the conduct of the pregnancy.
HER2/neu–targeted agents in pregnancy
Trastuzumab and lapatinib are two HER2/neu–targeted agents that have been used increasingly in recent years for the management of breast cancer. Zagouri and associates reviewed 18 pregnancies with in-utero exposure to trastuzumab. The mean duration of trastuzumab was 14.8 weeks and the most common adverse event was oligohydramnios or polyhydramnios, present in 61% of cases. Significant renal and pulmonary issues were present in 31.6% of neonates. All pregnancies with only first-trimester exposure resulted in healthy neonates. The authors conclude that brief exposure to trastuzumab early in pregnancy is unlikely to be detrimental, but should be avoided otherwise.
The increased risk of oligo- or anhydramnios is thought to result from the effect of trastuzumab on the fetal renal epithelium in which HER2/neu is strongly expressed. Some have correlated the effect of trastuzumab to an inhibition of the VEGF, which regulates the production and reabsorption of amniotic fluid. Prolonged exposure to trastuzumab was consistently associated with serious adverse events both on the pregnancy and on the fetus in these cases. If a patient is found to be pregnant while taking a HER2-targeted mAb, the drug should be discontinued, and the patient should be counseled that brief exposure to the drug is unlikely to be detrimental to the fetus and pregnancy. Elective administration of trastuzumab during pregnancy is not advisable.
The MotHER pregnancy registry was established in 2008 as a prospective, observational cohort study of women with breast cancer who were treated with trastuzumab, with or without pertuzumab or ado-trastuzumab emtansine during pregnancy or within 7 months of conception. The registry aims to describe pregnancy outcomes and complications. As of 2017, 20 women have enrolled and the results are pending.
Hormonal treatments for pregnancy-associated breast cancer
Preclinical models have shown that in utero exposure to tamoxifen increases the incidence of genital abnormalities. Tewari and colleagues reported the first case of fetal teratogenicity developing from maternal exposure to tamoxifen. The drug was discontinued at 20 weeks of gestation, but the fetus developed ambiguous genitalia with labial fusion and clitoromegaly. The largest known review of tamoxifen use in pregnancy reported on 167 patients, including 136 patients from AstraZeneca’s tamoxifen registry. The review reported an elevated risk of fetal anomalies compared to the general population (12.6% vs 3.9%). Anomalies included genital abnormalities and complex malformations.
As with trastuzumab, we recommend active contraception use during tamoxifen therapy, and in those cases in which pregnancy inadvertently occurs, the drug should be discontinued. Although 85 pregnancies with normal fetal outcomes are contained in a tamoxifen chemoprevention study, women should be counseled regarding the possibility of genital or more complex malformations associated with in utero exposure to this drug.
Prognosis of pregnancy-associated breast cancer
Thirty-two women with PABC referred to two European Union oncology centers between 1995 and 2007 were analyzed by Halaska and colleagues. Sixteen cases were diagnosed during pregnancy, and 16 were diagnosed within 1 year after delivery. The investigators matched each patient for age at diagnosis, tumor size, and stage to a control group of 32 nonpregnant patients. Overall survival was similar in the PABC and non-PABC patients ( P = .449). The subgroup of patients with PABC diagnosed within 1 year after delivery showed a shorter time to relapse than controls or patients in whom PABC was diagnosed during pregnancy ( P = .0178). Such findings concerning poorer prognosis among postpartum diagnoses had not been identified in previous series and warrant continued attention.
Cardonic and Iacobucci analyzed the maternal and fetal outcomes of 130 women with PABC that were reported to the voluntary Cancer and Pregnancy Registry and followed prospectively. Among the 130 patients, 120 were diagnosed with a primary tumor, 8 with recurrent disease, and 2 with a new primary malignancy. The mean maternal age at diagnosis was 34.8 years, and the mean gestational age at diagnosis was 13.2 weeks. For 113 women who were followed for a mean of 3.14 years, recurrence was reported in 30 women at a mean of 16.2 months from delivery. Twenty-one patients are deceased at a mean interval of 24.71 months from delivery to death. Only 42% were diagnosed with an ER-positive tumor, and 35% of cases had PR-positive disease. Human epidermal growth factor receptor 2 was positive in 25% of patients. Survival rates by stage for a primary diagnosis in pregnancy included stage I, 100%; stage II, 86%; stage III, 86%; and stage IV, 0%. These survival rates are similar to what is observed in the nonpregnant breast cancer population.
A very large study was conducted by Rodriguez and colleagues evaluating 797 PBAC cases identified by linking the California Cancer Registry (1991 to 1997) with the California Patient Discharge Data Set; 4177 age-matched, non-PBAC breast cancer control participants were also identified. PABC cases were significantly more likely to have more advanced-stage, larger primary tumor, hormone receptor–negative tumor, and mastectomy as a component of their treatment. In survival analysis, PABC had a higher death rate than non-PABC (39.2% vs. 33.4%; P = .002). In multivariable analysis, advancing stage, African American ethnicity (68% increased risk over non-Hispanic Whites), hormone receptor–negative tumors (20% increased risk), and pregnancy (14% increased risk) were all significant predictors of death.
Lactation
Whether breastfeeding is safe or possible has also been debated. Many have postulated that breast cancer is at least in part of viral origin, and the possibility exists that the contralateral breast will be contaminated with the causative agent, which will be passed to the fetus. This theory has never been borne out in fact, but most surgeons recommend artificial feeding of the infant, ostensibly to avoid vascular enrichment in the opposite breast, which may also contain a neoplasm.
Reports by Higgins and Haffty and by Tralins suggest that successful lactation in the breast treated by lumpectomy and irradiation is possible. Apparently, the location of the breast incision is important. It is not surprising that circumareolar incisions were associated with diminished ability to lactate because such incisions interrupt a large number of major milk ducts. Radial incisions in the breast interrupt fewer ducts but may be cosmetically inferior. In addition, the size, shape, and orientation of the nipple are important to allow its normal mechanical function. Patients whose nipples did not extend sufficiently, were not oriented properly, or were not supple found that the infant would not nurse from the treated breast. Finally, concerns have been expressed by some clinicians that attempts to breastfeed after conservative surgery may lead to a greater incidence of mastitis secondary to disruptions of the ductal system.
Hormonal considerations: Pregnancy preceding breast cancer
Protective effect of human chorionic gonadotropin.
The hormonal issues specific to breast cancer appear in Fig. 12.11 . Epidemiologic data have demonstrated a 50% reduction in the risk of breast cancer in women who complete full-term pregnancies before 20 years of age. The benefit is seen among all ethnic groups worldwide and increases with increasing parity. There is a declination in risk reduction beyond the age of 30 years. Both human and animal breast tissues and human breast cell lines contain low levels of receptors that bind hCG and its structural and functional homolog luteinizing hormone. These gonadotropins exert numerous anticancer effects in breast cancer models and cells, prompting some investigators to speculate that the elevated levels of hCG associated with full-term pregnancies may exert a protective effect against the later development of breast cancer.