Immune-based therapies for cancer are now commonplace. Cytokine therapy, including interferon and interleukin-2, is safe in the community setting. The US Food and Drug Administration has recently approved sipuleucel-T for the treatment of advanced prostate cancer, the first therapeutic cancer vaccine to meet this level of efficacy. The therapeutic use of monoclonal antibodies directed against proteins controlling various cell functions, including growth and modulation of immune response, has become so pervasive that the oncologist, whether surgeon or medical oncologist, must be familiar with indications, contraindications, and the associated toxicities.
On April 29, 2010, the US Food and Drug Administration (FDA) approved sipuleucel-T (Provenge) for the treatment of men with advanced, hormonally refractive, prostate cancer. With this, the notion of vaccines for the treatment of advanced cancer became a reality for individuals suffering from advanced prostate cancer. This breakthrough comes a century after the recognition by Coley that immunologic events, specifically infections, could result in regression of established cancer. Over the last century, the relationship between the immune system and the tumor environment has been explored on multiple levels with efforts to enhance immunity, tumor recognition, or both. These efforts are now making their way into the community with a number of immunologic agents approved by the FDA for ongoing use. For the purposes of this article, the authors focus on solid tumors and explore these immunologic approaches including cytokines, vaccines, antibody-based therapy, and cellular therapies—many of which are currently or will become common fare in community cancer programs.
Cytokines
The administration of high-dose, bolus interleukin (IL)-2 has consistently led to prolonged durable responses in patients with metastatic melanoma and kidney cancer. The demonstration by Rosenberg and colleagues of the efficacy of an immune modulating cytokine on established tumor clearly demonstrated the potential of immune-based therapies.
IL-2 is a 15.5 kD glycoprotein hormone with pluripotent immunomodulating properties including T cell activation that leads to downstream cascade and the release of other cytokines including IL-1, tumor necrosis factor, interferon-γ, IL-6, and lymphotoxin. These other cytokines may also contribute to the efficacy and side effects of IL-2 therapy and decrease after IL-2 treatment is completed.
Dose escalation trials were begun in the 1980s in patients with a variety of cancers. In a series of 25 patients, IL-2 resulted in significant responses in patients with advanced renal cell cancer (RCC) and in patients with refractory metastatic melanoma. Patients with metastatic RCC have historically had a median survival of 12 months and patients with melanoma 4 to 12 months. Large trials of patients with metastatic melanoma have shown a consistent response rate of 12% to 16%, with a 6% complete response rate. Trials of patients with refractory RCC have repeatedly demonstrated a response rate of 14% to 23% with 4% to 8% having a complete response. These responses have been remarkably durable, especially in patients who achieve a complete response. In both groups of patients, the median survival has not been reached. Work from the Cytokine Working Group and the authors’ data over the last decade have confirmed these findings.
The concern regarding the use of IL-2 stems from the significant toxicities associated with the administration of the high-dose regimen (720,000 IU/kg or 600,000 IU/kg intravenously every 8 hours). Most patients receive 6 to 10 doses in a cycle usually stopping due to dose-limiting toxicities or fatigue. Patients then return in 7 to 14 days for another cycle of therapy. If they have stable or responding disease, two additional cycles (also referred to as a course) are offered. A third course is usually limited to patients who have tolerated the treatment relatively well and have ongoing shrinkage of disease. This dosing schedule has been associated with a well described capillary leak phenomenon that routinely causes oliguria, mild hypoxemia, generalized edema, and hypotension. The expected toxicities are shown in Table 1 . Although these toxicities require hospitalization and dedicated support, they can be well managed with limited mortality. In our community teaching hospital, we have treated 196 patients with high-dose IL-2, administering 599 cycles of treatment with only one treatment-associated death.
| System | Toxicities | Percent IL-2 Courses in Which Grade 3 or 4 Toxicity Occurred N = 288 |
|---|---|---|
| Cardiovascular | Atrial or ventricular arrhythmias | 12.2 |
| — | Hypotension requiring pressors | 38.2 |
| Fatigue | Difficulty performing some activities | 1.4 |
| GI | Nausea and vomiting (severe) | 1.7 |
| — | Diarrhea (severe) | 1.0 |
| Hematologic | Low platelet count (<50,000) Low white blood count (<1,000) | 9.7 0.7 |
| Neurologic | Anxiety or depression (severe) | 1.4 |
| — | Confusion | 2.4 |
| Pulmonary | Shortness of breath (severe) | 1.0 |
| Renal | Elevated creatinine | 0.35 |
| — | Peripheral edema | 0.7 |
| — | Poor urine output | 6.3 |
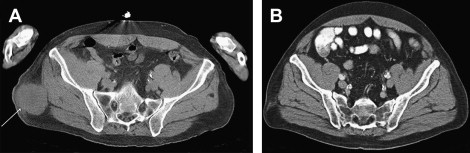
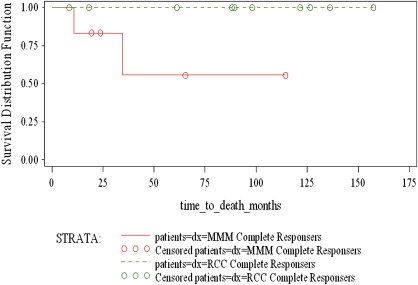
Significant responses can be achieved in patients with established metastases ( Figs. 1 and 2 ). Fig. 3 displays the survival curves of patients responding to IL-2 therapy. Table 2 details the response rates and durability of the responses. Based on the remarkable durability of the responses and the impact on a patient with a complete response, IL-2 was approved by the FDA in 1992 for use in patients with metastatic renal cell cancer and in 1998 for patients with advanced melanoma.
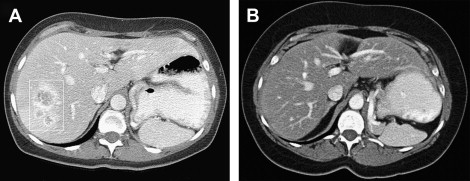
| Renal Cell Cancer | Melanoma | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Date | Number of Patients Treated | Response Rate (CR + PR) No. (%) | Ongoing Responses No. (%) | Duration of All Responses (Months) a | Number of Patients Treated | Response Rate (CR + PR) No. (%) | Ongoing Responses No. (%) | Duration of All Responses (Months) a |
| 1998 through 2009 | 86 | 23 (26.7) [CR = 6 (7%) PR = 17 (19.8%)] | 7 (8.1) | CR: 98+, 89+, 61+, 23, 18+, 8+ PR: 58+, 24, 23+, 22, 19, 17, 16, 15, 13, 11, 11, 10, 9, 7, 6, 5, 3 | 92 | 13 (14.1) [CR = 4 (4.3%) PR = 9 (9.8%)] | 7 (7.6%) | CR: 114+, 43, 24+, 19+ PR: 73+, 51+, 28+, 24+, 12, 8, 6, 5, 4 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree