Fig. 9.1
The ILC family members and their putative role in cancer. The ILC family comprises killer blood-circulating ILCs and helper-like tissue-resident ILCs. ILCs are further divided into three groups according their signature cytokines and their transcription factor requirement. The main characteristics of the three classes of ILCs are represented here, as well as their anti-tumor activity. Question marks (?) indicate possible roles that have not been demonstrated yet. Direct killing of tumor cells is well established for NK cells but only few studies have described ILC1-mediated cytotoxicity through the lytic granule pathway [13] or through TRAIL in the case of liver ILC1s [14]. Of note, only the protective roles of ILCs against tumors are depicted here
NK cells constitute the first ILC subset to be discovered and were initially identified for their spontaneous cytotoxic activity [19]. NK cells are often characterized as CD3 negative cells expressing CD56 in humans, NK1.1 in mice, and NKp46 in both species [20]. Of note, such phenotypic definition may include other ILC subsets. Moreover, NK cells are not a homogeneous population and can be divided into different subtypes [21]. In humans, the two main NK cell subsets are CD56brightCD16− NK cells which are important cytokine producers and are abundant in lymph nodes, and CD56dimCD16+ NK cells which represent the main NK cell population in the blood and are highly cytotoxic [22]. NK cell-depleting antibodies as well as mouse models displaying NK cell deficiencies have been fundamental for the demonstration of NK cell anti-cancer activity (Table 9.1). NK cells harbor many surface receptors allowing them to distinguish malignant-transformed cells from healthy cells. Moreover, NK cells are endowed with potent cytotoxic functions and are a major source of the anti-tumor cytokine, IFN-γ. These characteristics make NK cells key protagonists of innate immunosurveillance of cancers [29]. In neat opposition to the numerous reports on NK cells in cancer, the possible pro- or anti-tumor functions of helper-like ILCs remain largely unexplored [30]. Nonetheless, recent work suggested an involvement of tissue-resident type 1-like ILCs in the immune surveillance of spontaneous tumors [13]. Furthermore, various reports described tumor-suppressive activities of ILC2s [31, 32] and ILC3s [33, 34].
Table 9.1
Mouse models and depleting antibodies used to investigate NK cell functions
Model | Characteristics | References |
|---|---|---|
Anti-NK1.1 Abs | These Abs deplete NK cells and NKT cells in C57BL/6 mice but do not deplete NK cells in mouse strains that do not express NK1.1 such as BALB/c mice | [23] |
Anti-AsialoGM1 Abs | These Abs deplete NK cells but not NKT cells. Anti-AsialoGM1 Abs may affect other cell populations and have been found to deplete basophils | [24] |
Beige mice | Beige mice have a defect in granulation and exhibit severe NK cell deficiency but also display defects in other granulocytes, cytotoxic T cell responses, and antibody responses | [25] |
Rag−/−γc −/− mice | Compared with Rag−/− mice that lack T and B lymphocytes, Rag−/−γc −/− mice lack both innate and adaptive lymphocytes. Indeed, Rag−/−γc −/− mice lack the common gamma chain (γc or Il2rg) that is required for IL-7 and IL-15 signaling and thus essential for ILC development | [26] |
NKDTR/EGFP transgenic mice | These mice express the diphtheria toxin receptors under the NKp46 promoter. Diphtheria toxin injection in these mice leads to NK cell depletion and may also deplete other NKp46+ ILCs | [20] |
Mcl1fl/flNcr1-Cre mice | These mice lack NK cells and other NKp46+ ILCs | [27] |
Ncr1greenCre Il2rg fl/fl | These mice lack all NKp46+ ILCs | [28] |
In this chapter, we will review the different mechanisms by which ILCs detect malignant cells and prevent cancer development. Most of our current knowledge is restricted to NK cells which constitute the prototypical anti-cancer ILC subset. Therefore, we will predominantly focus on NK cells while introducing emerging data on helper-like ILCs. It should be noted that both pro- and anti-tumor activities of helper-like ILCs have been described [35] but herein we will only discuss their potential host protective functions.
9.2 Surface Receptors Involved in Tumor Recognition by ILCs
Innate cells express a fixed set of germline-encoded receptors that allow the recognition of foreign, aged and damaged cells [36]. Activating and inhibitory surface receptors are crucial for the regulation of NK cell functions and some of these receptors are also expressed on helper-like ILCs subsets (Fig. 9.2). Furthermore, interactions with accessory cells, generally monocytes or dendritic cells (DCs), also stimulate NK cells to produce pro-inflammatory cytokines and potentiate their killing functions [37]. Historically, NK cells were described for their ability to kill tumor cells having down-regulated MHC class I molecules (MHC-I), a concept termed “missing-self” recognition [38]. Most cell types express self-peptide-MHC-I complexes on their surface but partial or complete loss of MHC-I expression is a common feature of cancer cells [39]. This phenomenon is often caused by CD8+ T cell-mediated immune pressure and renders tumor cells susceptible to NK cell-mediated cytotoxicity. Conversely, MHC-I molecules expressed at the surface of healthy autologous cells bind to inhibitory NK cell receptors and deliver negative signals thereby avoiding NK cell autoreactivity. Besides the “missing-self” recognition, the detection of stress-induced self-ligands expressed at the surface of damaged cells also promotes NK cell killing capacities. In fact, the outcome of NK cell interaction with a target cell is determined by the balance between inhibitory signals transmitted by NK cell receptor binding to self MHC-I and activating signals transmitted upon recognition of stress ligands at the surface of the target cell [40]. Activating NK cell receptors involved in the immunosurveillance of cancers include the natural cytotoxicity receptors (NCRs), NKG2D (also known as CD314 and KLRK1) and DNAM-1 (also known as CD226) [36]. Other receptors such as the low-affinity activating receptor FcγRIIIa (CD16) or the co-stimulatory molecules CD137, OX40, and GITR are promising clinical targets for their ability to mediate potent NK cell activation [41]. Nonetheless, these receptors have not been reported to play any role in the early detection of nascent tumor and therefore will not be discussed here.
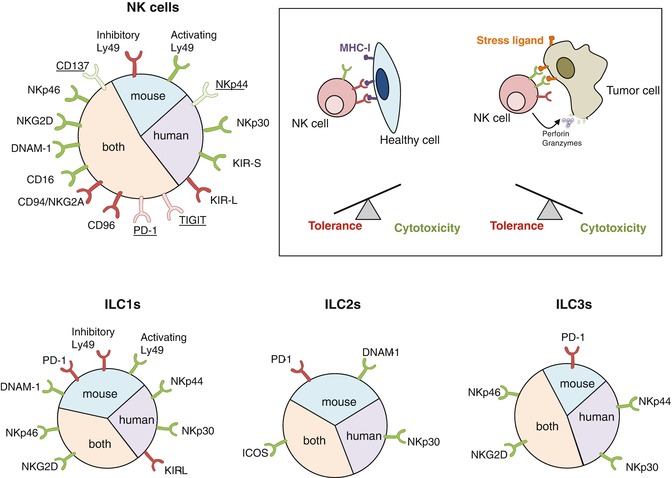

Fig. 9.2
Cell surface receptors involved in tumor cell recognition by ILCs. NK cells express an array of activating (green) or inhibitory (red) cell surface receptors. Some receptors are only expressed on activated NK cells (underline). The outcome of NK cell interactions with a target cell is determined by the balance between activating and inhibitory signals. Healthy cells express MHC-I molecules that engage NK cell inhibitory receptors whereas cancer cells down-regulate MHC-I molecules and/or express stress ligands recognized by NK cell activating receptors. An excess of activating signals over inhibitory signals leads to NK cell activation and the cytotoxicity of the target cell. Helper-like ILCs also express receptors that may regulate the sensing of tumor cells and some of these receptors have been found to regulate cytokine production by helper-like ILCs. DNAM-1 and PD-1 have recently been observed on mouse ILCs but their expression on human ILCs has not been investigated yet
There are three members of the NCR family: NKp46 (NCR1; CD335) is expressed in both mice and humans, whereas NKp44 (NCR2, CD336) and NKp30 (NCR3; CD337) are restricted to human NK cells [42]. Unlike NKp46 and NKp30, NKp44 is not detected on resting NK cells but is up-regulated after activation. NCR engagement triggers NK cell-mediated cytotoxicity and secretion of IFNγ [36]. Importantly, NCR expression is not restricted to NK cells and is shared with helper ILC1s [16], a subset of human ILC2s [43] as well as a subset of ILC3s named NCR+ ILC3s [10]. Engagement of NKp44 by tumor cells and tumor-associated fibroblast stimulates NCR+ ILC3 to release IL-8 and TNF [33]. Similarly, NKp30-mediated recognition of human tumor cell lines induces the NF-κB signaling pathway in ILC2s, leading to the production of IL-13 and other type 2 cytokines [43]. Whether NCRs also govern helper-like ILC1 recognition of malignant cells is yet to be demonstrated. NCR ligands on tumor cells have only been partially defined and those reported include NKp44L, HLA-B associated transcript 3 (BAT3), B7-H6, heparan sulfates, and proliferating cell nuclear antigen (PCNA) [42]. Curiously, PCNA differs from other NCR ligands as it does not stimulate but rather inhibits NK cell functions [44]. Interestingly, NCR genes encode different splice variants, some of them being immunosuppressive. A recent report suggested that the cytokine-defined microenvironment may influence NKp30 and NKp44 isoform expression profile in NK cells and that alternative splicing gives rise to inhibitory isoforms that dampen NK cell functions [45]. Furthermore, in gastrointestinal stromal tumors, predominant expression of the immunosuppressive NKp30c isoform over the immunostimulatory NKp30a and NKp30b isoforms is associated with reduced survival [46]. Remarkably, mice lacking NKp46 have been useful to define NKp46 involvement in the control of initial tumor growth [47] and the prevention of tumor metastasis [48] in vivo. Unfortunately, in vivo investigation of the other NCRs in tumor immunosurveillance is limited by the lack of mouse orthologs for NKp30 and NKp44.
NKG2D is a major NK cell activating receptor also expressed by some T cell subsets [36]. NKG2D recognizes several MHC-related ligands that are poorly expressed at the surface of healthy cells but are frequently up-regulated with the process of malignant transformation [49]. For instance, in non-transformed mouse or human cells, activation of the DNA-damage response induces the up-regulation of NKG2D ligands and enhances cellular sensitivity to NK cell killing [50]. NKG2D ligands are RAE-1α-ɛ, MULT1 and H60a-c in mice; and MICA-B and ULBP1–6 in humans. NKG2D engagement stimulates signaling cascades leading to cell activation, killing, and cytokine production. A pioneer study demonstrated that mouse tumor cell lines engineered to express high levels of NKG2D ligands and injected subcutaneously into syngeneic mice are rapidly rejected by conventional NK cells without a requirement for T and B cells [51]. Notably, the pivotal role of NKG2D in tumor immunosurveillance has been evidenced in mouse models of de novo tumorigenesis [52] and carcinogenesis [53]. In a model where expression of the Epstein–Barr virus transforming protein LPM1 in mouse B cells led to the development of B cell lymphomas, the arising lymphoma cells expressed ligands for NKG2D and were killed in vitro by NK cells [54]. However, in this model, T cells were the major effectors of immunosurveillance. It is possible that arising lymphomas may have developed escape mechanisms to circumvent NK cell anti-tumor activity in vivo. In fact, tumor progression is usually associated with an immunoediting process resulting in the emergence of malignant clones that are resistant to NK cell activity [55]. This has been illustrated in multiple myeloma where the transition from a pre-malignant to a malignant stage of the disease is associated with shedding of MICA from the surface of the tumor cells [56]. In addition to reducing NKG2D ligand surface density on tumor cells, this shedding process generates soluble ligands that down-regulate NKG2D expression on immune cells and promote tumor immune evasion [57]. Actually, chronic exposure to low-affinity surface-attached NKG2D ligands also leads to NK cell desensitization to both NKG2D-dependent and -independent pathways [58]. Surprisingly, instead of blocking tumor cell recognition, shedding of the high affinity NKG2D ligand MULT1 promotes tumor rejection by boosting NK cell effector functions [59]. It was suggested that MULT1 prevents immunosuppressive interaction with low-affinity ligands such as RAE-1 expressed in the tumor microenvironment and restores NK cell responsiveness.
DNAM-1 is an adhesion molecule expressed on NK cells and T cells that associates with the integrin LFA-1 and participates to the stabilization of the cytolytic synapse [60]. DNAM-1 recognizes a the family of nectin and nectin-like molecules initially identified for their role in cell-cell adhesion [61]. Nectin and nectin-like molecules have been involved in a wide range of biological processes and they notably regulate the immune functions of T cells, NK cells, and antigen presenting cells. In addition to enhancing NK cell adhesion and cytotoxicity, DNAM-1 promotes the secretion of IFN-γ. Moreover, DNAM-1 expression distinguishes two functional NK cell subsets in mouse [62]. Compared with DNAM-1− NK cells, DNAM-1+ NK cells have enhanced IL-15 signaling and produce higher levels of pro-inflammatory cytokines. Intriguingly, DNAM-1 is expressed at high levels on mouse liver ILC1s [17] and is also detected on early ILC precursors as well as ILC2 progenitors in the mouse bone marrow [63]. Still, the putative role of DNAM-1 in the regulation of helper-like ILC function remains to be investigated. DNAM-1 ligands, CD155 (also known as PVR) and CD112 (also known as nectin-2, PRR2, or PVRL2) are often over-expressed by solid and hematological malignancies [60]. Similarly to what has been described for NKG2D ligands, DNA-damage resulting from replication stress seems responsible for tumor cell expression of the DNAM-1 ligand CD155, and this pathway is dependent on ATM, an enzyme that senses double strand DNA breaks [64]. An interesting study performed in the Eμ-Myc mouse lymphoma model established that the DNA-damage response induces CD155 expression on early stage transformed B cells and thereby leads to spontaneous tumor regression that is partially DNAM-1-dependent [65]. Further strong evidence of DNAM-1 contribution to immunosurveillance comes from the observation of enhanced development of carcinogen-induced fibrosarcomas [66] as well as accelerated growth of spontaneous and transplantable tumors in DNAM-1-deficient mice [67, 68].
The variety of activating receptors expressed by NK cells is complemented by numerous inhibitory receptors that prevent the killing of healthy tissue. Receptors binding to self-MHC-I are responsible for the “missing-self” recognition. NK cell receptors of the KIR family in humans and of the Ly49 family in mice directly recognize MHC-Ia molecules [36]. Moreover, the CD94/NKG2A heterodimeric receptor is expressed in both species and binds to a peptide presented by the non-classical MHC molecule HLA-E in humans and Qa-1 in mice. Successful engagement of MHC-I by these receptors transmits an inhibitory signal that disrupts activating pathways. For instance, engagement of the inhibitory receptor KIR2DL2 blocks activating receptor clustering and induces actin remodeling and concomitant retraction from the target cell [69]. The importance of the missing-self recognition in NK cell-mediated immunosurveillance has been highlighted by the report of accelerated onset of carcinogen-induced sarcomas and spontaneous B cell lymphomas in mice expressing reduced levels of Ly49 inhibitory receptors [70]. So far, NK cells are considered the sole ILC subset mediator of the “missing-self” recognition. Notwithstanding, Ly49 receptors have been detected on the surface of other ILCs subsets [13, 16] but their function needs to be assessed.
Additional receptors may regulate ILC functions in the tumor microenvironment. The immune checkpoint molecules CTLA-4 and PD-1 have been found to hinder NK cell activity and constitute important clinical targets [41] but little is known about their role in innate immunosurveillance of cancer. Interestingly, mouse precursors for helper-like ILCs have been characterized by high expression levels of PD-1 [63]. Furthermore, few mature ILCs express PD-1, but they up-regulate this immune checkpoint molecule upon activation [71]. More investigation would be required to assess the role of PD-1 on ILCs within tumors. Besides, two receptors interacting with the nectin and nectin-like molecule family, TIGIT and CD96 (also known as TACTILE), have recently gained clinical interest for their potent inhibition of NK cell- and T cell-functions [72]. TIGIT and CD96 bind to CD155 and counterbalance DNAM-1-mediated activation of NK cells [60]. TIGIT inhibits mouse and human NK cell-mediated cytotoxicity [73, 74] and CD96 was shown to reduce mouse NK cell production of IFN-γ [75]. CD96-deficient mice display robust resistance to experimental lung metastasis and carcinogenesis [75], but the role of TIGIT in NK cell-mediated surveillance of cancers remains to be established.
9.3 Cytokines and Soluble Factors that Activate ILCs
Besides cell-to-cell interactions, ILCs integrate multiple signals provided by soluble mediators such as cytokines, alarmins, lipids, or hormones produced by epithelial, stromal, or myeloid cells [76] (Fig. 9.3). Tumor growth is likely to disturb the homeostasis of the surrounding tissue, leading to the release of cytokines and danger signals that might shape ILC-mediated immunosurveillance. Both NK cells and helper-like ILC1s are responsive to IL-12, IL-15, and IL-18, while ILC2s mainly respond to IL-25, IL-33, and TSLP; and ILC3s are activated by IL-23 and IL-1β. There is little direct evidence of the role of physiologically secreted cytokines on ILCs in cancer and most studies have rather investigated the effect of exogenous cytokine administration or used genetically manipulated tumors or mice. For example, a report described the resistance of transgenic mice over-expressing IL-15 to subcutaneously injected B16 melanoma cells lacking MHC-I [77]. In this study, the anti-tumor activity was maintained in the absence of CD4+ or CD8+ T cells, but protection was lost upon NK cell depletion with anti-asialo-GM1 antibodies. Moreover, IL-2, IL-12, and IL-18 promote NK cell-mediated control of experimental metastasis in mice [78, 79] and NK cells pre-activated with IL-12, IL-15, and IL-18 display sustained effector functions and delay the growth of MHC-I-deficient tumors in mice [80]. Intriguingly, a more recent study established that the IL-12-induced suppression of subcutaneous B16 melanoma tumors was mediated by a NKp46+NK1.1− ILC3 population identified using a fate-mapping reporter mouse strain for the transcription factor RORγt [34]. Finally, the expression of Toll-like receptors by NK cells and ILC3s [81, 82] might enable these cells to detect danger-associated molecular patterns (DAMPs) present in the tumor microenvironment. DAMP may induce NK cell activation either directly, or indirectly through the stimulation of accessory cells [37]. Interestingly, a recent study established that NK cells from TLR3-deficient mice are hyporesponsive to cytokine stimulation, a defect that is associated with increased experimental lung metastasis in these mice upon challenge with B16F10 melanoma cells [83].
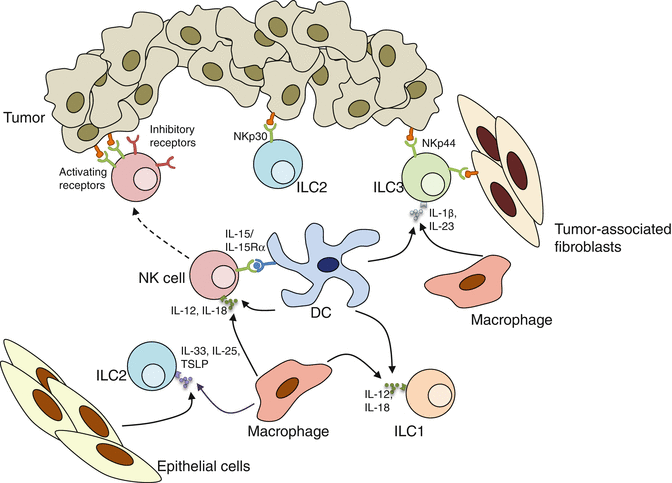

Fig. 9.3
Cytokines and membrane-bound ligands contribute to ILC activation. Cytokines such as IL-12, IL-15, and IL-18 produced by DCs and macrophages prime NK cells increase their capacities upon engagement of activating receptors. IL-12 and IL-18 also stimulate IFN-γ production by ILC1s. ILC2s respond to IL-25, IL-33, and TSLP. In addition, human ILC2s are activated upon NKp30 engagement, while human ILC3s respond to NKp44 stimulation. Moreover, ILC3s are activated by the cytokines IL-1β and IL-23
Notably, helper-like ILCs are mainly activated via soluble mediators while NK cell receptors represent a predominant pathway for NK cell activation. Nevertheless, cytokine-activated NK cells are more responsive to NK cell receptor signaling than resting NK cells. NK cell-activating cytokines are generally secreted by myeloid cells or dendritic cells (DCs) [84]. In fact, it has been suggested that naïve NK cells only acquire their full killing capacity following an interaction with DCs or macrophages termed “NK cell priming” [85], a process that is influenced by the commensal microbiota [86]. Of particular interest, similar synergy between cytokine-mediated activation and NCR signaling has recently been described for NCR+ ILC3s [87].
9.4 Direct Clearance of Cancer Cells by ILCs
The release of cytotoxic granules containing perforin and granzymes constitutes the main pathway by which NK cells exert their killing activity [88]. Perforin is a pore-forming protein that allows granzymes to enter into target cells, thereby triggering apoptosis [89]. Perforin protects mice against spontaneous lymphomas [90] and mouse studies have established the major contribution of perforin to NK cell-mediated rejection of tumor lacking MHC-I [91, 92], as well as NK cell-mediated control of metastasis [23] and protection against carcinogen-induced fibrosarcomas [93]. Granule-dependent cytotoxicity was originally thought to be a characteristic of NK cells distinguishing them from ILC1s [12]. However, it was recently demonstrated that a population of ILC1-like cells clearly distinct from conventional NK cells could also kill tumor cells in a perforin-dependent fashion [13]. Notably, this study used a spontaneous mouse mammary cancer model to suggest that ILC1-like cells, but not conventional NK cells, contribute to reduce tumor growth.
Fas ligand (FasL or CD95L) and TNF-related apoptosis inducing ligand (TRAIL) belong to the death receptor pathway and represent alternative mechanisms by which NK cells eliminate target cells [14, 94, 95]. The binding of Fas or TRAIL to their receptors (Fas and DR5 or DR4, respectively) triggers the activation of the common death signaling molecules FADD, caspase 8, and caspase 3, and leads to apoptosis [96]. The relevance of targeting Fas- and TRAIL-death receptor pathways to bypass the refractory nature of cancer stem cells to conventional therapy has been demonstrated in mice [97]. Initially, TRAIL-positive NK cells were described in the liver of naïve mice; and in vitro killing activity of hepatic but not splenic NK cells was found to be TRAIL-dependent [14]. Accordingly, TRAIL appears necessary for the control of experimental liver metastasis in mice [14]. Interestingly, TRAIL is largely expressed by immature NK cells in new-born mice and is required for fetal NK cell killing activity of TRAIL-sensitive targets in vitro [98]. Similarly, TRAIL is required for the in vitro killing activity of human cord blood NK cells whereas the cytotoxicity of mature human NK cells mostly relies on the perforin and FasL pathways [99]. While the origin of TRAIL+ fetal NK cells remains unclear, mouse liver TRAIL+ NKp46+ cells have now been assigned to the ILC1 lineage [100]. In the healthy human liver, NK cells do not express TRAIL but its expression can be induced by pro-inflammatory cytokines [101]. It was recently shown that TRAIL up-regulation is confined to a specific population of human intra-hepatic NK cells that express CXCR6 and are absent from the periphery [102]. Thus, in the absence of inflammatory stimuli, TRAIL expression might be restricted to tissue-resident subsets of the group 1 ILCs. As a matter of fact, in mice, TRAIL has also been detected on salivary gland ILC1s [18] and tumor-infiltrating ILC1-like cells [13]. However, the observation that cytokine stimulation induces TRAIL expression on CD3−NK1.1+ cells in the murine spleen [103] and CD3−CD56+ cells in the human blood [104] suggests that TRAIL also contributes to conventional NK cell functions under some circumstances. In fact, membrane-bound TRAIL supplements perforin-mediated killing of neuroblastoma and multiple myeloma cell lines by activated NK cells isolated from human peripheral blood [105, 106]. Additional investigation should shed light on the respective roles played by blood-circulating conventional NK cells and tissue-resident ILC1s in the TRAIL-mediated control of nascent tumors. In opposition to TRAIL, there is very limiting data supporting a role of FasL in NK cell-mediated-control of tumors in vivo [107]. Noteworthy, a recent study elegantly demonstrated that IL-18 induces a rapid expression of FasL on the surface of mouse NK cells and NK cell-mediated FasL-dependent cytotoxicity was found to control MC38 liver metastases in mice [108].
An important characteristic of group 1 ILCs is the secretion of IFN-γ and TNF [109]. These two cytokines play a major role in tumorigenesis. Not only do they modulate immune responses, but they also directly impact on tumor cell biology. Actually, enhanced in vivo growth of various mouse cell lines has been observed upon ablation of tumor-responsiveness to IFN-γ [110]. Responses to IFN-γ are induced by the JAK-STAT signaling pathway. In cancer cells, this pathway has been shown to inhibit cellular proliferation and to promote apoptosis [110]. The central role played by endogenously produced IFN-γ in promoting the immune-mediated elimination of nascent tumor cells has been demonstrated by Schreiber and colleagues [111]. An early study indicated that the combination of perforin and IFN-γ pathways fully accounts for NK cell anti-metastatic activity in mice [112]. Nonetheless, since IFN-γ can be produced by many different innate and adaptive immune cell types, the formal proof of ILC contribution to IFN-γ-mediated immunosurveillance is still lacking. As for TNF, the role of this cytokine in cancer biology is rather ambiguous [113]. The two receptors to TNF are TNFR1, which is expressed on all cell types and TNFR2, which expression is restricted to immune and endothelial cells. Paradoxically, TNFR1 can transmit both pro-survival and pro-apoptosis signals. As a result, some reports described cytostatic or cytotoxic effects on tumor cells while others observed an enhancement of malignant cell proliferation (for a review, see [113]). The observation of reduced in vitro killing capacity of NK1.1+ splenocytes from TNF-deficient mice against YAC-1 suggested that TNF contributes to NK cell-mediated killing [114]. Furthermore, NK cell cytotoxicity against chemotherapeutic-sensitized mouse MC38 tumors was found to be TNF-dependent [115]. But overall, to date there is no convincing evidence of a major contribution of TNF to innate cell-mediated cytotoxic activity. In fact, spleen cells from TNF-deficient mice are perfectly able to lyse MHC-I deficient RMA-S tumor cells in vitro [116]. However, defective elimination of RMA-S cells was observed following intraperitoneal injection in TNF-deficient mice [116]. This phenomenon was explained by reduced NK cell accumulation in the peritoneum in the absence of TNF. Moreover, TNF-neutralization inhibits NK cell activation and thus reduces human NK cell-mediated cytotoxic activity against myeloma cells in the presence of anti-CD319 mAbs (Elotuzumab) [117]. Collectively, these data indicate that albeit not directly cytotoxic, TNF secreted by NK cells might act to regulate the tumor microenvironment.
Of note, although direct cytotoxic activity toward tumor cells is considered as a specificity of group 1 ILCs, ILC2s were recently found to induce tumor cell apoptosis via the CXCR2 pathway [32].
9.5 Cross-Talk between ILCs and Other Immune Cells Resulting in Anti-Cancer Immunity
NK cell functions expend far beyond the simple killing of cancer cells [118]. In addition to IFN-γ and TNF, activated NK cells release a broad range of cytokines, including GM-CSF, IL-6, and IL-10, and they may facilitate the recruitment of other immune cells by secreting chemokines such as MCP-1 (CCL2), MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), IL-8 (CXCL8), and IP-10 (CXCL10) [119]. IFN-γ production by NK cells has been shown to promote macrophage-mediated immunoediting of carcinogen-induced tumors in mice [120]. Moreover, NK cells influence the outcome of developing T cell responses in many different ways [121]. For instance, NK cells enhance the priming cytotoxic CD8+ T cell responses by eliminating myeloid-derived suppressor cells [122] and activating DCs [123, 124]. Tumor cell killing by NK cells and the subsequent release of antigen further contributes to T cell priming [125]. Besides, NK cells recruited to inflamed lymph nodes provide an early source of IFN-γ necessary for Th1 polarization of CD4+ T cells [126]. The direct cytotoxicity of activated regulatory T cells (Tregs) represents another mechanism by which NK cell could potentiate effector T cell responses [127]. Importantly, in some mouse tumor models, NK cells were found to contribute to the priming of tumor-specific memory T cells required for the long-term control of cancer [123, 128].
Our understanding of helper-like ILC mediated-regulation of anti-tumor responses is still in its infancy. Given the important role of IFN-γ in NK cell-mediated tuning of adaptive immune responses, it is tempting to hypothesize that IFN-γ production by ILC1s would contribute in a similar manner. However, there is currently no study supporting this statement. In fact, the evaluation of the relative contribution of NK cells and ILC1s to anti-tumor immunity is complicated by the high phenotypic resemblance of these cells. Of note, TRAIL+ members of the group 1 ILC might also dampen T cell responses [129]. Regarding ILC2s, the type 2 cytokines produced by these cells usually inhibit type 1 anti-tumor responses and foster tumor progression [9]. Nevertheless, in the mouse B16F10 model of experimental metastasis, IL-5 production by lung ILC2s cells has been shown to promote eosinophil recruitment and clearance of lung tumors [31]. A positive role of ILC3s in anti-tumor immunity was suggested in a study where combined treatment of chemotherapy with tumor-targeting antibodies resulted in delayed growth of B16 subcutaneous tumors [130]. In this setting, tumor clearance was found to be dependent on CD90+NK1.1−RORγt+ innate lymphocytes and was associated with increased infiltration of macrophages within the tumor tissue. Another report demonstrated that NKp46+ ILC3s suppress the growth of subcutaneously injected B16F10 tumor cells engineered to secrete IL-12 [34]. IL-12-secreting tumors were still repressed in the absence of T cells or of conventional NK cells. It was suggested that NKp46+ ILC3s mediated their anti-tumor functions by up-regulating adhesion molecules on the tumor endothelium. Similarly, the production of soluble factors by NCR+ ILC3s present in human non-small cell lung cancer (NSCLC) tissues was found to activate mesenchymal stem cells and endothelial cells [33]. In this study, ILC3 numbers within the tumor tissue were found to correlate with the density of TLS tertiary lymphoid structures (TLS) which are ectopic lymphoid organs associated with a favorable prognosis in NSCLC patients [131]. Very interestingly, lower frequencies of tumor-infiltrating NCR+ ILC3s were observed in advanced tumors, suggesting that NCR+ ILC3s might be associated with a better prognosis for NSCLC patients [33].
Conclusions
ILCs act as sentinels that react promptly upon disturbance of host homeostasis [76]. Their rapid and robust response allows the temporary control of the danger and alerts other immune cells to provide long-term protection (Fig. 9.4). NK cells have long been known as the most powerful innate guardians against cancer development. However, the recent expansion of the ILC family may challenge this idea and raises the question of the relative contribution of the different ILC subsets, in particular NK cells and ILC1s. Noteworthy, despite considerable transcriptomic overlap with ILC1s, NK cells express higher transcripts encoding proteins of the cytotoxic machinery as well as cell surface receptors involved in the detection of transformed cells [100]. Moreover, helper-like ILCs are tissue-resident cells [132] that would only sense alterations of the specific organ where they are located whereas conventional NK cells circulate in the blood and can scan the whole body for the presence of damaged cells. Thus, NK cells are probably the most efficient ILC subset in tumor clearance.
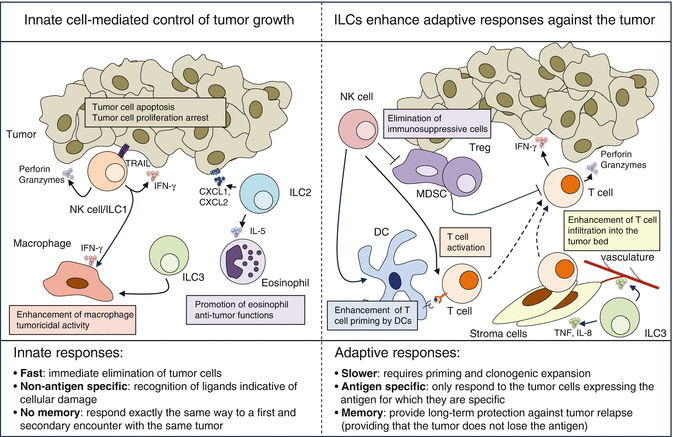

Fig. 9.4
Innate and adaptive lymphocytes contribute to cancer immunosurveillance. Left panel: ILCs directly induce tumor cell apoptosis and/or growth arrest and they also stimulate other innate cells to ensure the fast clearance of cancer cells. Group 1 ILCs exert a direct cytotoxic activity against tumor cells either via the cytotoxic granule pathway (perforin/granzymes) or the death ligand pathway (TRAIL). Of note, such cytotoxic activity has been described for both conventional NK cells and ILC1s but NK cells are currently considered more potent killers. IFN-γ production by group 1 ILCs also directly inhibits tumor growth and may stimulate macrophage tumoricidal activity. ILC2s might directly induce the apoptosis of CXCR2-expressing tumor cells via the secretion of CXCL1 and CXCL2. ILC2s also secrete IL-5 and thereby recruit eosinophils. ILC3s may increase macrophage infiltration into the tumor. Right panel: ILCs stimulate T cell responses and thereby ensure long-term protection against cancer cells. NK cells activate DCs, enhance anti-tumor T cell responses, and also kill immunosuppressive cells such as Tregs and myeloid-derived suppressor cells (MDSC). ILC3s activate stroma and endothelial cells and thereby facilitate immune cell infiltration into the tumor bed
The importance of ILCs in the presence of functional adaptive immunity was recently questioned as ILC deficiency occurring in a cohort of SCID patients appeared to have no major clinical consequences [133]. This study provided a 7–39 year follow-up of 18 patients with mutation of IL2RG or JAK3 treated with hematopoietic stem cell transplantation in the absence of myeloablation. However, this cohort was too small to address the role of ILCs in tumor immunosurveillance and such investigation may also require a longer follow-up. Perhaps the most convincing evidence of the importance of cytotoxic ILCs for human cancer immunosurveillance comes a prospective study demonstrating that individuals with high spontaneous cytotoxic activity of peripheral blood lymphocytes were at significantly lower risk of developing cancers [134]. In gastrointestinal sarcoma patients, NK cell infiltration correlates with the absence of metastasis at diagnosis [46] and in renal cell carcinoma, high densities of NK cells in lung metastases are associated with prolonged survival [135]. Conversely, a study in NSCLC reported no correlation between NK cell numbers and clinical outcome [136]. An absence of NK cell activity against advanced cancers may be caused by (1) tumor escape from NK-cell immunosurveillance due the selection of resistant variant clones through the immunoediting process [120] and/or (2) the exhaustion of NK cells within the tumor microenvironment where a variety of mechanisms contribute to hinder NK cell functions [55]. Adenosine and TGF-β are two examples of soluble factors proven to affect NK cell activity against tumors [137, 138]. Furthermore, poor NK cell infiltration in human cancer tissues could explain their limited impact on solid tumor progression. However, NK cells seem particularly efficient against metastatic disease [27] and hematological malignancies [139].
Evidence for helper-like ILC subsets involvement in human cancers is limited. ILC3 infiltration has been observed in human colorectal cancer [140], primary tumors of breast cancer patients [141], and NSCLC tissues [33]. Importantly, the function of helper-like ILCs in malignant diseases remains unclear and may depend on the cancer type and stage. This chapter is focused on the protective role of ILCs in the detection and elimination of nascent tumors and the reader is invited to refer to other reviews for a complete discussion of the opposing abilities of helper-like ILCs to either promote or repress tumor growth [9, 30, 35]. It is possible that the tumor microenvironment hijacks ILCs, either by dampening ILC anti-tumor activity such as IFN-γ release, or by influencing ILC plasticity. Indeed, some ILC subsets are not stable and depending on the cytokine microenvironment, NK cells can acquire an ILC1 phenotype [18], ILC2s can convert into ILC1s [142] or ILC3s [143], and ILC3s can convert into ILC1s [144]. The hypothesis that tumor may escape immunosurveillance by modulating ILCs is supported by the report that both ILC functions and subtype composition are dysregulated in the blood of acute myeloid leukemia patients [145].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree