Fig. 1
Developing a prostate cancer chemoprevention cocktail. Cancer is as diverse as the individual is and therefore needs a personalized rather than a generalized approach. Also, carcinogenesis is a multistep process; it is unlikely that single-agent approach could prove effective in preventing cancer. In search for safe and nontoxic chemopreventive agents, many naturally occurring bioactive food components, capable of affording protection against carcinogenesis, have been defined. Thus, the new effective approach for cancer prevention “building a customized mechanism-based chemoprevention cocktail of naturally occurring substances” is advocated
2 Diet in Causation and Prevention of Cancer
Diet is a complex mixture of chemicals and thus for cancer risk is a mixed bag of bad and good stuff, carcinogens and anticarcinogens, respectively. Current evidence for the involvement of diet in cancer etiology is based on convincing laboratory data where dietary manipulations in rodent tumor bioassay protocols have established a definite link. Much of the knowledge of the role of diet in human cancer outcome however is based on indirect relationships between the consumption of selected food constituents and dietary habits and incidence of cancer at various sites. The indirect evidence, most often referred to, is the suggested correlation between the complex of fats-meat-egg-animal protein and the risk for cancer of various organs (Modan 1977). Carcinogenic agents identified include food additives, plant toxicants, aflatoxins, polycyclic hydrocarbons, nitrosamines, and certain normal major food constituents (Modan 1977). A synergistic action of ingested or metabolized carcinogens and a co-carcinogenic function of certain dietary components are suggested. Abundant epidemiological, observational, and metabolic biomarker studies have provided convincing evidence that nutrition plays an important causative role in the initiation, promotion, and progression stages of several types of human cancers [(Mukhtar and Ahmad 1999) and the references therein]. It has become clear that, in addition to substances that pose a cancer risk, the human diet also contains agents which are capable of affording protection against some forms of cancer.
Over last two decades there has been a constant increase in the awareness and utilization of diet as complementary and alternative medicine for cancer control. This increase is in part due to the fact that anything “natural” is considered inherently safe. Chemoprevention, by definition, is “the use of drugs, vitamins, or other agents to try to reduce the risk of, or delay the development or recurrence of, cancer.”. A recent task force on chemoprevention defines the approach as “the prevention of cancer or treatment of identifiable precancers” (Kelloff et al. 2006; Herberman et al. 2006). The expectation of chemoprevention at the cellular level is regulation of growth and differentiation, at the tissue level is the reversal of premalignant lesions, and at the clinical level it is nothing less than reduction of cancer development and outcome. Thus, the achievable goal of chemoprevention could be defined as “slowing the process of carcinogenesis” (Adhami and Mukhtar 2013). Chemoprevention of cancer thus differs from cancer treatment in that the goal of this approach is to lower the rate of cancer incidence. Chemopreventive agents are often, but erroneously, also called as anticarcinogens. Chemoprevention in recent years is increasingly realized as a promising approach for cancer control because the therapy and surgery have not been fully effective against the high incidence or low survival rate of most of the cancer types including PCa (Herberman et al. 2006; Santillo and Lowe 2006). Micronutrients present in edible plants are regarded as the most desirable class of chemopreventive agents (Santillo and Lowe 2006). This information is supported by the fact that epidemiological studies suggest that consumption of fresh fruits and yellow-green vegetables reduces the cancer incidence and mortality due to stomach, colon, breast, lung, bladder, esophageal, prostate, and other cancers (Adhami et al. 2003; Khan et al. 2009; Wattenberg et al. 1990; Kellof et al. 1994). A wide range of such micronutrients present in plants consumed by humans has been shown to possess potential chemopreventive effects. Some of the well-identified chemopreventive agents, in addition of possessing preventive effects, are also showing therapeutic potential (Ames 1983; Greenwald 2002), and often they enhance the therapeutic efficacy of established chemotherapeutic agents. At the present time, about 30 classes of chemicals with such effects have been described, many of which may have practical implications in reducing cancer incidence at least in high-risk individuals (Tsao et al. 2004; Ren and Lien 1997; Surh 2003). Among the large list of chemopreventive agents, the polyphenolic antioxidants present in a variety of plant foods and beverages consumed by the human population are receiving increasing attention for human consumption (Lall et al. 2015; Mukhtar and Ahmad 1999; Santillo and Lowe 2006; Ren and Lien 1997; Surh 2003).
3 Prostate Cancer: An Ideal Disease for Chemoprevention
PCa represents an excellent candidate disease for chemoprevention because it is a unique malignancy which generally grows very slowly, likely for decades, before symptoms arise and a diagnosis is finally established. It is typically diagnosed in elderly men and, therefore, even a modest delay in the neoplastic development achieved through pharmacological or therapeutical intervention could result in substantial reduction in the incidence of the clinically detectable disease (Surh 2003; Klein and Thompson 2004). Consistent with this assumption, there is intense activity in defining chemopreventive agents and molecular targets for PCa chemoprevention [(Adhami et al. 2003) and references therein]. Among many such agents, for a variety of reasons naturally occurring nontoxic dietary substances are preferred. Our studies supported by the data from other laboratories worldwide suggest that there are multiple targets for PCa chemoprevention by naturally occurring agents and therefore highlight the need for further studies to identify novel pathways that may be modulated by dietary agents that could be further exploited for prevention and treatment of PCa.
It will be important to investigate which nutritional intervention could lead to a reduction in the incidence of PCa in what population and in what stage of the disease. Thus, efforts are under way to define agents that can delay the conversion of prostatic intra-epithelial neoplasia to well-differentiated adenocarcinoma, a delay in the conversion of well-differentiated adenocarcinoma to moderately differentiated adenocarcinoma, or a delay in the conversion of moderately differentiated adenocarcinoma to poorly differentiated adenocarcinoma (Brawley and Parnes 2000). Below we describe our experience with some of the agents and define the molecular targets of observed preventive effects.
4 ODC and Prostate Cancer
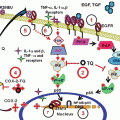
The enzyme ornithine decarboxylase (ODC) is a homodimer of 461 amino acids that catalyzes the decarboxylation of ornithine, producing as a result diamine putrescine. This is the first step and the rate-limiting step in humans for the production of polyamines which is required for cell growth, proliferation, and differentiation (Abrahamsen et al. 1991; Auvinen et al. 1992). Convincing evidence is there to provide the role of polyamines in tumor cell growth and in biological response of tumor promoters and growth factors (Pegg et al. 1995; Meyskens and Gerner 1999). Various rodent studies have established the importance of ODC in tumor progression [(Meyskens and Gerner 1999) and references therein; (Lan et al. 2000)].
In humans, among all tissues, the highest concentration of polyamines and polyamine synthesis enzymes, especially ODC, occurs in the prostate (Mohan 1999). We, thus, hypothesized that ODC could serve as a biomarker for the diagnosis or monitoring the therapeutic efficacy of PCa in human, and possibly as a target for intervention of the disease through chemoprevention. In our first set of experiments, we demonstrated that as compared to benign tissue ODC enzyme activity is elevated 2.7-fold in PCa tissue, PCa tissue has highly expressed ODC protein, and ODC enzyme activity is highly elevated in prostatic fluids of patients with PCa (Mohan 1999). In one of our subsequent study, we observed that ODC is upregulated in transgenic adenocarcinoma of the mouse prostate (TRAMP) prostate model (Gupta et al. 2000a). TRAMP is an excellent mouse model of PCa that mimics progressive forms of human disease inasmuch as 100 % of males develop histological PIN by 8–12 weeks of age that progresses to adenocarcinoma with distant site metastases by 24–28 weeks of age. We then found that oral feeding of α-difluoromethylornithine (DFMO), a suicide substrate inhibitor of ODC, results in the inhibition of prostate carcinogenesis and its metastasis in TRAMP mice (Gupta et al. 2000a). DFMO has repeatedly been suggested to play a key role in the prevention of PCa in both in vivo and in vitro situations [(Kadmon 1992) and the references therein]. Clinical trials with ODC inhibitors in PCa patients have yielded encouraging results (Simoneau et al. 2001). A large clinical study has demonstrated that the administration of oral DFMO for 4 weeks reduces the levels of putrescine, spermidine, and spermine in a statistically significant manner in human prostate tissue (Hikosaka et al. 2004). Many dietary chemopreventive agents are known to inhibit ODC induction in various test systems. A recent study observed that the inhibitory effects of soy isoflavones on rat prostate carcinogenesis induced by PhIP are mediated by downregulation of ODC (Hikosaka et al. 2004). Thus, some of the dietary substances could be explored for their role in inhibiting PCa development by inhibiting ODC activity.
5 COX-2 and Prostate Cancer
Prostaglandin (PG) endoperoxidase synthase, commonly referred to as cyclooxygenase (COX), is a key enzyme involved in the conversion of arachidonic acid to PGs and other eicosanoids. COX exists in two isoforms, namely, COX-1 and COX-2 with distinct tissue distribution and physiological functions. COX-1 is the housekeeping enzyme, constitutively expressed in many tissues and cell types and is involved in normal cellular physiological functions, whereas COX-2 is the inducible isoform, is pro-inflammatory in nature, and is inducible by mitogens, cytokines, tumor promoters, and growth factors. Under laboratory conditions, induction of COX-2 has been shown to promote cell growth, inhibit apoptosis, and enhance motility and adhesion (Williams et al. 1999; Cao and Prescott 2002). Although there has not been a common consensus on the association of COX-2 with disease stage, it is generally agreed that COX-2’s overexpression is associated with development of various cancers including cancers of prostate and colon. In several types of cancer, overexpression of Cox-2 is correlated with advanced diseases and poor prognosis (Hikosaka et al. 2004; Williams et al. 1999; Cao and Prescott 2002). In the search for agents that will prevent or delay the inception of cancer, it is realized that COX-2 is a promising therapeutic target (Pruthi 2004). We, in a study, have shown that COX-2 mRNA and protein levels were overexpressed in PCa tissue compared with paired benign tissue. Immunohistochemical analysis also verified COX-2 overexpression in cancer tissues (Gupta et al. 2000b). Many other studies have verified this initial observation and reported that as compared to normal tissue, COX-2 is overexpressed in human PCa. In this scenario, it has been suggested that selective inhibition of COX-2 may be useful for prevention and/or therapy of PCa [(Gupta et al. 2004) and the references therein].
Epidemiological studies and clinical observations suggest that nonsteroidal anti-inflammatory drugs (NSAIDs) and certain selective COX-2 inhibitors may reduce the relative risk of clinically evident PCa. NSAIDs are amongst the most commonly used medications worldwide. They are considered as effective anti-inflammatory, antipyretic, and analgesic drugs. Studies involving NSAIDs and PCa suggest that NSAIDs have a preventive effect on PCa. A multicentric cohort of over 90,000 men demonstrated a 24 % reduction in the risk for PCa in patients receiving aspirin daily in year prior to the study (Habel 2002). Other relatively large case–control studies demonstrated even more significant reduction in the risk of PCa (Roberts et al. 2002; Leitzmann et al. 2002; Irani et al. 2002; Royle and Ross 2004). NSAIDs, in spite of being effective against PCa have limited application due to severe toxic side effects on normal cells [(Irani et al. 2002) and the references therein]. Therefore, novel nontoxic COX-2-specific inhibitors that have the ability to spare normal cells from their cytotoxic effects are required for cancer chemoprevention. The efficacy of celecoxib, a selective COX-2 inhibitor which reduces inflammation and side effects associated with traditional NSAIDs, was tested in TRAMP mice on the progression of PCa by measuring the growth of primary tumor and effects on distant site metastases, the intermediate, and end point markers of PCa progression (Gupta et al. 2004). Celecoxib supplementation in the diet to TRAMP mice resulted in significant reduction in tumor development with no signs of metastasis. Celecoxib fed animals showed reduced proliferation and downmodulation of COX-2 and prostaglandin E2 levels in dorsolateral prostate and plasma. These results correlated with retention of anti-metastasis markers, viz., E-cadherin, and α- and β-catenin along with significant decrease in VEGF protein expression. Celecoxib supplementation also resulted in enhanced in vivo apoptosis in prostate as monitored by 99mTc-labeled annexin V in live animals followed by phosphor imaging. It is noteworthy that many other diet-based cancer chemopreventive agents also inhibit COX-2 induction. Hussain et al. (2005) recently demonstrated that EGCG inhibits COX-2 without affecting COX-1 expression at both the mRNA and protein levels, in androgen-sensitive LNCaP and androgen-insensitive PC-3 human prostate carcinoma cells. More studies are also needed for the identification of signaling pathways and specific genes being modulated by the Cox-2-derived prostaglandins in tumorigenesis. These studies will shed light on prostate cancer development and will also help design new targeted therapy for this disease.
6 Green Tea and Prostate Cancer
Various epidemiological studies have indicated that people who regularly consume tea have a decreased risk of PCa (Heilbrun 1986; Jain et al. 1998; Jian et al. 2004). A case–control study in southeast China suggested that green tea is protective against PCa (Jian et al. 2004). According to the study, the incidence rate of PCa per 100,000 is 104.33 in the USA and 75.97 in Australia but only 1.74 in China possibly because of use of green tea and other natural products. In a recent clinical trial, Bettuzzi et al. (2006a) observed that green tea catechins (GTC) when given to patients with high-grade prostate intra-epithelial neoplasia significantly decreased the tumor incidence to ~3 % in GTC-treated men as compared to 30 % in placebo-treated men.
More than a decade ago, our lab initiated a program to assess whether green tea consumption could afford chemopreventive effects against PCa development. Since then we have been able to assess multiple targets by which tea affords PCa chemopreventive effects. At first, we showed that ODC, a rate-controlling enzyme in the polyamine biosynthesis pathway, is overexpressed in prostate cancer and prostate fluid in humans (Gupta et al. 1999). High testosterone levels are known to mediate the induction of ODC activity and exposure of PCa cells to Epigallocatechin-3-Gallate (EGCG), and infusion of green tea to Cpb: WU rats caused a downregulation of ODC activity. Green tea and its individual components have been found to modulate a variety of pathways involved in the pathogenesis of PCa.
Studies from ours and other laboratories have shown that EGCG results in an induction of apoptosis in PCa cells (Ahmad et al. 1997; Gupta et al. 2000c; Paschka et al. 1998). One of the additional study from our laboratory demonstrated that EGCG-induced apoptosis in human prostate carcinoma cells is mediated via modulation of two related pathways: p53 and NF-κB (Hastak et al. 2003). Yu et al. (Yu et al. 2004) demonstrated that the addition of EGCG and Cu2+ to the growth medium decreased the relative viability of human PCa cells. In a study from our laboratory, EGCG was also found to increase the expression of cell cycle regulatory molecules p21, p16, and p18 while downmodulating the protein levels of cyclin D1, cyclin E, cdk2, cdk4, and cdk6 (Gupta et al. 2003).
We then reasoned that the preclinical studies should be carried out in a model system that mimics the PCa development in a similar fashion to human disease. TRAMP is one such model and we have provided convincing evidence that oral infusion of green tea polyphenols (GTP) (equivalent to six cups of green tea human consumption) to the TRAMP mice inhibits prostate carcinogenesis through the modulation of IGF/IGFBP-3 signaling pathway (Gupta et al. 2001). In a subsequent study, we demonstrated that IGF/IGFBP-3 signaling is the prime pathway for GTP-mediated inhibition and metastasis of PCa in TRAMP mice that limits the progression of cancer through inhibition of metastasis and angiogenesis markers, most notably vascular endothelial growth factor (VEGF), urokinase plasminogen activator (uPA), and matrix metalloproteinases (MMPs) (Adhami et al. 2004).
Studies have also demonstrated the effects of tea and its polyphenols on the growth of prostate tumor xenografts. Subsequent to the work of Liao et al. (1995), we in a recent study demonstrated that GTP, black tea extract, EGCG, and the aflavin (polyphenol found in black tea) administration resulted in a significant inhibition in the growth and development of PCa cells implanted in nude mice and that this inhibition was accompanied with reduced serum prostate specific antigen (PSA) levels and an induction of apoptosis and inhibition of angiogenesis (Siddiqui 2006).
Green tea has been explored in the clinic for its anticancer effects. Studies that recruited patients at an advanced stage of the disease reported limited effect, suggesting that green tea could be more effective if given to patients at high risk for developing cancer. Bettuzzi et al. (2006b) observed that after a year of green tea administration, only one man in a group of 32 with high-grade PIN developed prostate cancer compared with 9 of 30 in the placebo group. A 24-month follow-up of patients from the same study suggested that the effects of green tea supplementation were long lasting (Brausi et al. 2008).
7 Pomegranate and Prostate Cancer
The fruit pomegranate, derived from the tree Punica granatum, has been shown to possess strong antioxidant, anti-inflammatory, antiatherogenic, and anti-tumorigenic properties (Gil et al. 2000; Afaq et al. 2005; Aviram and Dornfeld 2001). In fact, the antioxidant activity of pomegranate fruit is shown to be higher than that of red wine and green tea, two dietary substances, which are showing promise in preclinical PCa models and in PCa patients (Gil et al. 2000).
In a recent study, employing highly aggressive human PCa cells, we observed that pomegranate fruit extract (PFE) treatment resulted in inhibition of cell growth mediated through induction of apoptosis (Malik et al. 2005). We also observed a significant inhibition in the growth and development of PCa cells implanted in nude mice which was also accompanied with concomitant reduction in serum PSA levels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree