Venous thromboembolic events are common in patients with malignancy, producing both morbidity and mortality. Although the underlying mechanisms by which cancer might promote a procoagulant state are multifaceted and incompletely understood, recent translational research has significantly advanced our understanding of the relationship between cancer and thrombosis. The occurrence of a thromboembolic event in a patient with an active malignancy should be regarded as a life-threatening complication, and decisions regarding prevention and treatment are usually complex. Outcomes may be improved by an evidence-based approach to management with consideration of practice guidelines.
The pathophysiology of the prothrombotic state of cancer is complex and multifactorial, resulting from a detrimental combination of intrinsic tumor cell properties, therapeutic interventions, and prolonged severe illness, which promote venous thromboembolic events through direct interactions with coagulation pathways, venous stasis, and endothelial injury ( Fig. 1 ). Investigators have identified many possible molecular explanations by which tumor cells might affect the coagulant potential of the blood, such as increased levels of inflammatory cytokines (eg, tumor necrosis factor α and interleukin-1) and oncogene-mediated alterations in the levels of important proteins such as plasminogen activator inhibitor 1 (PAI-1). Three of the most studied candidates (tissue factor, cancer procoagulant, and mucin) are discussed here.

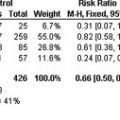
Tissue factor (TF), a transmembrane glycoprotein, is the primary cellular initiator of the extrinsic coagulation pathway, and is most notably expressed on the surface of subendothelial cells, platelets, and cell-derived microparticles. Tumor cells in a wide variety of malignancies overexpress TF on their surface and in association with microparticles; increased expression of TF appears to be associated with a significantly increased incidence of thrombotic events. In one study of 41 patients with pancreatic cancer, immunohistochemical staining showed that higher TF expression was associated with venous thromboembolism (VTE) (odds ratio, 4.5).
Cancer procoagulant (CP) is a 68-kDa vitamin K–dependent protein found exclusively in amniotic-chorionic tissues and malignant cells. In ex vivo studies, CP directly activates factor X in the absence of factor VII and has been shown to activate platelets with a mechanism similar to thrombin. CP has been identified in the serum of 85% of cancer patients.
The increased risk of thrombotic events associated with mucinous adenocarcinoma is well known. This phenomenon may in part result from the emission of abnormal mucins into the circulation; mucins interact with leukocytes and platelets through circulating L- and P-selectin molecules. These interactions lead to the activation of platelets, causing the formation of thrombi.
In addition to the cellular mechanisms described in the preceding paragraphs, multiple extrinsic factors contribute to increased risk of venous thromboembolic events in cancer patients. For example, chemotherapy, radiation, surgery, direct compression of a blood vessel by a bulky tumor, immobilization, and placement of central venous catheters can all play a role in the formation of a pathologic clot. Although a number of chemotherapy agents predispose to thrombosis, antiangiogenic drugs such as thalidomide (Thalomid), lenalidomide (Revlimid), and bevacizumab (Avastin) may pose an especially high risk for patients who receive them in combination with corticosteroids or additional chemotherapy. Recently, erythropoiesis-stimulating agents such as epoetin (Epogen, Procrit) and darbopoetin (Aranesp) have also been associated with malignancy-associated VTE.
Primary prevention of venous thromboembolism in cancer patients
In selected, high-risk cancer patients, thromboprophylaxis has the potential to improve outcomes. Hospitalization increases the risk of VTE in cancer patients. Multiple large, randomized controlled studies have found that use of low-molecular-weight heparins (LMWH) or fondaparinux (Arixtra) significantly reduces the incidence of VTE in patients hospitalized for acute medical illness without increasing the incidence of adverse events compared with placebo. A meta-analysis of multiple randomized controlled trials (RCTs) enrolling a heterogeneous group of “medically ill” inpatients suggests that unfractionated heparin (UFH) has efficacy and safety profiles comparable to LMWH. Unfortunately, no studies of VTE prevention specifically in cancer patients admitted for medical reasons have been performed.
Both the American Society of Clinical Oncology (ASCO) and the American College of Chest Physicians (ACCP) recommend that hospitalized cancer patients be considered as candidates for pharmacologic VTE prophylaxis in the absence of bleeding or other contraindications. Provider compliance with thromboprophylaxis guidelines is particularly low in cancer patients, perhaps because thrombocytopenia and other bleeding risk factors are common in these patients. The platelet count below which the risk of “prophylactic-dose” heparin or LMWH outweighs the benefit is not known. Institution-based alert systems may increase physician compliance with pharmacologic prophylaxis, and reduce the incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized patients.
The utility of thromboprophylaxis in ambulatory cancer patients has been investigated in multiple clinical trials. A recent trial randomized 1150 ambulatory patients with metastatic or locally advanced cancer to receive thromboprophylaxis with nadroparin (Fraxiparine) versus placebo while undergoing treatment with chemotherapy. A significant reduction in VTE was observed (from 3.9% to 2.0%), although the overall effect was small (absolute risk reduction 1.9%; number needed to treat 52.6). Interim analysis of 2 RCTs investigating chemoprophylaxis with LMWH in patients with advanced pancreatic cancer receiving chemotherapy demonstrated a significant decrease in the rate of both VTE and death in this population. Current practice guidelines do not recommend the use of thromboprophylaxis for ambulatory cancer patients, with the exception of those undergoing treatment with thalidomide or lenalidomide in combination with high-dose steroids or chemotherapy.
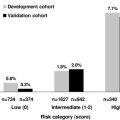
Although studies support the use of pharmacologic prophylaxis in patients with cancer, the unanswered question is: in which patients can the risk and inconvenience of primary VTE prophylaxis be justified? The identification of such a subgroup will be difficult in clinical practice, but a risk-stratification model developed by Khorana and colleagues (for chemotherapy-associated thrombosis) may be helpful; the model uses readily available clinical and laboratory data to divide patients into groups at low, intermediate, or high risk for VTE. The high-risk group developed symptomatic VTE at a rate of approximately 6.7% over a median time of 2.5 months. This was much higher than the 0.3% of patients in the low-risk group who developed VTE ( Fig. 2 ).

Compared with patients without cancer who undergo major surgery, cancer patients are at increased risk (both magnitude and duration) of developing postoperative venous thromboembolic events. VTE is the most common cause of death during the first 30 days after major oncologic surgeries. Advanced age, late-stage malignancy, prolonged anesthesia, protracted time of immobilization, and prior history of VTE are associated with increased risk of postsurgical VTE.
Well-designed trials have shown that LMWH and UFH effectively reduce the incidence of VTE in patients undergoing major abdominal or pelvic surgery for malignancy without increasing the risk of adverse events. A systematic review and meta-analysis of 14 RCTs involving 3986 patients with cancer found no differences in mortality when using LMWH versus UFH for perioperative thromboprophylaxis. Although definitive evidence is not yet available, at least 2 studies have found that higher doses of LMWH are more efficacious and equally safe for preventing VTE when compared with lower doses of LMWH in surgical cancer patients. A randomized controlled trial in patients undergoing high-risk abdominal surgery found fondaparinux to have equal efficacy and safety when compared with dalteparin for VTE prophylaxis. Post hoc analysis of patients with cancer revealed possible superior efficacy of fondaparinux in this subpopulation but this finding needs confirmation in a prospective trial. Consensus guidelines recommend UFH, fondaparinux, or LMWH as VTE prophylaxis for cancer patients undergoing major surgery.
The results of more than one clinical trial indicate that prolonged use of LMWH (4 weeks) compared with short-term use (1 week) for thromboprophylaxis in patients who have undergone pelvic or abdominal surgery for cancer reduces the risk of venographically detected VTE, and does not carry an increased risk of bleeding or adverse events. Although a recent systematic review highlights the need for more evidence of net benefit, both the ACCP and ASCO guidelines suggest 4 weeks of LMWH (extending past the period of hospitalization) for “higher risk” patients.
Neurosurgical interventions in patients with cancer are associated with high rates of venous thromboembolic events. Pharmacologic prophylaxis has traditionally been avoided in these patients because of concerns about intracranial bleeding. A small RCT found that postoperative LMWH combined with graduated compression stockings significantly decreases the incidence of VTE when compared with compression stockings alone, without increasing the risk of intracranial bleeding. The ACCP guidelines recommend UFH or LMWH combined with mechanical prophylaxis in all high-risk neurosurgery patients.
Gynecologic surgery for malignancy also carries a considerable risk for thromboembolic complications. Studies have shown that UFH significantly reduces DVT in this group of patients, when compared with no prophylaxis, and that LMWH has a similar efficacy and safety profile to UFH in this group. The ACCP recommends UFH or LMWH, combined with mechanical prophylaxis, in all patients undergoing surgery for gynecologic malignancy, and 4 weeks of LMWH extended past the hospitalization for higher risk patients.
Treatment of VTE in cancer
Pharmacologic anticoagulation is highly effective in preventing complications of VTE as well as recurrent events. A strategy of initial anticoagulation with LMWH, fondaparinux, or intravenous (IV) UFH followed by long-term targeted therapy with an oral vitamin K antagonist (VKA) is widely used to treat acute VTE in the general population. However, the long-term use of oral VKAs in cancer patients is less effective and less safe when compared with patients without cancer. For the initial treatment of VTE, LMWH is preferred in cancer patients because it can be used in an outpatient setting, does not require laboratory monitoring, and has a lower risk of heparin-induced thrombocytopenia. Furthermore, a recent systematic review suggests that LMWH may be more effective than UFH in this setting. The efficacy of fondaparinux is comparable to heparin or LMWH in initial treatment of VTE for patients without cancer; however, there is a lack of cancer-specific evidence to support its use for the treatment of malignancy-associated VTE.
The largest RCT comparing long-term treatment (ie, secondary prevention) of VTE in cancer patients found LMWH was more efficacious and had a similar rate of bleeding complications when compared with a VKA. A total of 672 patients were randomized to receive monotherapy with dalteparin, or dalteparin followed by a coumarin for 6 months. Twenty-seven (9%) of 336 patients who received monotherapy with dalteparin had a recurrent VTE at 6 months compared with 53 (17%) of 336 patients in the control group, a relative risk reduction of 52%. Compared with VKA treatment, the risk of major bleeding was not increased by long-term therapy with LMWH. A recent Cochrane Collaboration systematic review confirmed the superiority of LMWH compared with VKAs in the secondary prevention of VTE in cancer patients. Practice guidelines produced by the ACCP, National Cancer Center Network (NCCN), and ASCO recommend treatment with LMWH for 3 to 6 months following initial VTE and indefinitely if active cancer or other risk factors persist.
VTE (usually pulmonary embolism) may present in an otherwise asymptomatic cancer patient as an incidental finding on routine imaging; the prevalence of these unsuspected thrombi is 1.9% to 6.3% in patients undergoing CT scan for staging purposes. A recent retrospective-cohort study compared mortality in 104 cancer patients with symptomatic VTE, 37 cancer patients with asymptomatic VTE, and 48 cancer patients with no VTE. All patients with symptomatic or asymptomatic VTE received anticoagulation with UFH or LMWH. Six-month mortality in the groups with symptomatic and asymptomatic VTE was similar (48.6% and 51.0%) and was significantly higher than the group with no VTE (27.1%). Although the impact of anticoagulant therapy on mortality or VTE recurrence risk in cancer patients who have asymptomatic VTE is unknown, ACCP practice guidelines recommend therapeutic anticoagulation comparable to what would be used for cancer patients whose VTE is symptomatic.
Inferior vena cava (IVC) filters have a limited role in the treatment of cancer-associated VTE. When added to anticoagulation therapy in VTE patients without cancer, placement of permanent IVC filters reduces the incidence PE from 15.1% to 6.2%; however, the incidence of DVT increases and there is no overall survival benefit at 8 years. Evidence from cancer-specific studies investigating use of permanent or retrievable IVC filters for treatment of VTE is lacking. IVC filter placement is appropriate for cancer patients with symptomatic VTE who have contraindications to anticoagulation.
Treatment of VTE in cancer
Pharmacologic anticoagulation is highly effective in preventing complications of VTE as well as recurrent events. A strategy of initial anticoagulation with LMWH, fondaparinux, or intravenous (IV) UFH followed by long-term targeted therapy with an oral vitamin K antagonist (VKA) is widely used to treat acute VTE in the general population. However, the long-term use of oral VKAs in cancer patients is less effective and less safe when compared with patients without cancer. For the initial treatment of VTE, LMWH is preferred in cancer patients because it can be used in an outpatient setting, does not require laboratory monitoring, and has a lower risk of heparin-induced thrombocytopenia. Furthermore, a recent systematic review suggests that LMWH may be more effective than UFH in this setting. The efficacy of fondaparinux is comparable to heparin or LMWH in initial treatment of VTE for patients without cancer; however, there is a lack of cancer-specific evidence to support its use for the treatment of malignancy-associated VTE.
The largest RCT comparing long-term treatment (ie, secondary prevention) of VTE in cancer patients found LMWH was more efficacious and had a similar rate of bleeding complications when compared with a VKA. A total of 672 patients were randomized to receive monotherapy with dalteparin, or dalteparin followed by a coumarin for 6 months. Twenty-seven (9%) of 336 patients who received monotherapy with dalteparin had a recurrent VTE at 6 months compared with 53 (17%) of 336 patients in the control group, a relative risk reduction of 52%. Compared with VKA treatment, the risk of major bleeding was not increased by long-term therapy with LMWH. A recent Cochrane Collaboration systematic review confirmed the superiority of LMWH compared with VKAs in the secondary prevention of VTE in cancer patients. Practice guidelines produced by the ACCP, National Cancer Center Network (NCCN), and ASCO recommend treatment with LMWH for 3 to 6 months following initial VTE and indefinitely if active cancer or other risk factors persist.
VTE (usually pulmonary embolism) may present in an otherwise asymptomatic cancer patient as an incidental finding on routine imaging; the prevalence of these unsuspected thrombi is 1.9% to 6.3% in patients undergoing CT scan for staging purposes. A recent retrospective-cohort study compared mortality in 104 cancer patients with symptomatic VTE, 37 cancer patients with asymptomatic VTE, and 48 cancer patients with no VTE. All patients with symptomatic or asymptomatic VTE received anticoagulation with UFH or LMWH. Six-month mortality in the groups with symptomatic and asymptomatic VTE was similar (48.6% and 51.0%) and was significantly higher than the group with no VTE (27.1%). Although the impact of anticoagulant therapy on mortality or VTE recurrence risk in cancer patients who have asymptomatic VTE is unknown, ACCP practice guidelines recommend therapeutic anticoagulation comparable to what would be used for cancer patients whose VTE is symptomatic.
Inferior vena cava (IVC) filters have a limited role in the treatment of cancer-associated VTE. When added to anticoagulation therapy in VTE patients without cancer, placement of permanent IVC filters reduces the incidence PE from 15.1% to 6.2%; however, the incidence of DVT increases and there is no overall survival benefit at 8 years. Evidence from cancer-specific studies investigating use of permanent or retrievable IVC filters for treatment of VTE is lacking. IVC filter placement is appropriate for cancer patients with symptomatic VTE who have contraindications to anticoagulation.
Central venous catheter–related thrombosis in cancer patients
Central venous catheters (CVC) are commonly used in cancer patients. Infection and VTE complicate their use. The incidence of catheter-related thrombosis in cancer patients is variable; in the largest prospective study to date, 4.3% of patients developed symptomatic CVC-associated DVT. Upper extremity DVTs associated with CVC can result in significant morbidity and mortality. Monreal and colleagues prospectively observed 86 non–CVC-associated upper extremity DVTs in patients who did not have cancer: 13 patients were diagnosed with PE, and 2 died from PE despite adequate therapy with intravenous heparin. Another prospective study followed 53 patients with upper extremity DVT who did not have cancer, 27.3% of whom developed post-thrombotic syndrome at 2 years. CVC-related thrombosis usually develops in the ipsilateral subclavian vein, innominate vein, or rarely the superior vena cava within 4 to 8 weeks following placement of a central venous catheter. It may occur less frequently with tip placement at the junction of the superior vena cava and the right atrium.
The use of anticoagulation for thromboprophylaxis in patients with long-term indwelling catheters has been investigated in multiple RCTs; meta-analyses and systematic reviews of the available evidence yield conflicting results. A recent meta-analysis of 9 randomized, prospective trials evaluated the efficacy of thromboprophylaxis in patients with cancer. Data from 852 patients who received heparin (UFH or LMWH) showed a nonsignificant reduction of symptomatic DVT (relative risk [RR], 0.43; 95% confidence interval [CI], 0.18–1.08), with no effect on mortality (RR, 0.74; 95% CI, 0.40–1.36) or major bleeding (RR, 0.68; 95% CI, 0.10–4.78). In the 1007 patients who received warfarin (or no treatment), no statistically significant impact on the risk of CVC-associated thrombosis was seen (RR, 0.62; 95% CI, 0.30–1.27). Current ACCP, NCCN, and ASCO guidelines do not recommend routine thromboprophylaxis in cancer patients with indwelling CVCs.
There is insufficient evidence to make strong recommendations about the treatment of catheter-related thrombosis in cancer patients. In one nonrandomized, prospective study, 46 patients (34 with cancer) with upper extremity DVT were anticoagulated with dalteparin for a minimum of 5 days, followed by long-term targeted warfarin therapy (international normalized ratio [INR]: 2.0–3.0). At 12 weeks, 1 patient had recurrence of DVT and 1 patient had a significant bleed. ACCP recommendations for treatment of upper extremity DVT are similar to guidelines for treating lower extremity DVTs, as no large randomized trials have compared efficacy, intensity, or duration of therapy with patients in this population. In response to the lack of expert recommendations addressing the treatment of catheter-related thrombosis in cancer patients, a multidisciplinary working group established by the French National Federation of Cancer Centers recently published guidelines according to the Standards, Options, and Recommendations methodology (SOR). The SOR guidelines recommend treating CVC-associated thrombosis in cancer patients with prolonged use of LMWH, and that long-term anticoagulation with a VKA should be reserved for patients with severe renal impairment.
Removal of the catheter in patients with CVC-associated upper extremity DVT should be considered but is not obligatory. The benefit of line removal was examined (along with other treatment modalities) in a retrospective study involving 319 cancer patients, of whom 112 had CVC-related thrombosis identified with radionucleotide imaging. All patients received one or more of the following interventions: warfarin, line removal, or line replacement. Regardless of the intervention, no major complications such as PE or death occurred. All patients in the study, except 4 who had been treated with line replacement, had complete resolution of their presenting symptoms. Another clinical trial treated 74 patients with active malignancy and CVC-associated upper extremity thrombosis with dalteparin and warfarin. After 3 months of treatment, no patients had recurrence of VTE, and no CVCs had been removed because of line failure or VTE recurrence/extension. The most recent guideline published by the ACCP does not recommend removal of an indwelling catheter if the device is functioning, and there is an ongoing need for the catheter. The SOR guidelines state that maintenance of the catheter is justified in the event that the catheter is mandatory, functional, in the right position, not infected, and showing a favorable evolution under close monitoring.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree