8 Cancer and Nutrition
8 Cancer and Nutrition
 Introduction
Introduction
Many components of our diet either protect against cancer or cause cancer themselves. There is now compelling scientific evidence of a causal relationship between many nutritional factors and certain types of cancer. So far, about 700 chemical compounds are known to induce cancer in animal experiments (16).
Despite recent advances, the options for cancer treatment remain quite limited. Hence, prevention plays an important role—especially in the context of nutrition.
In a joint effort, the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) have compiled a report entitled “Food, Nutrition, and the Prevention of Cancer: a Global Perspective,” in which they reviewed and analyzed the available scientific evidence on the topic of nutrition and the prevention of cancer in the mid-1990s (107). They have concluded that a healthy nutrition combined with physical exercise and avoiding becoming overweight may reduce the incidence of cancer by 30–40%. This amounts to three to four million individuals with cancer every year worldwide.
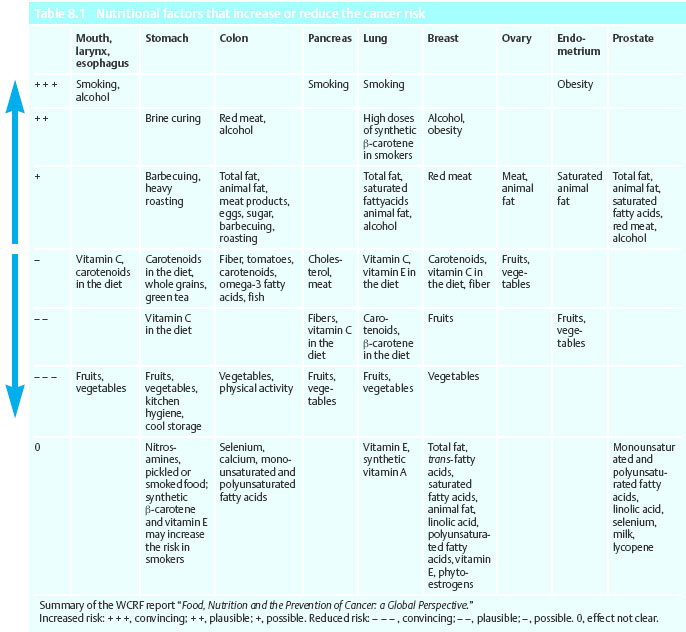
Table 8.1 provides an overview of the assessment of the correlation between certain nutritional factors and specific types of cancer. It is striking to see that both fruits and vegetables play a prominent role in the prevention of cancer. For almost every type of cancer, there is evidence of protective nutritional factors. Among the cancer-promoting factors, obesity plays a major role in addition to smoking and alcohol. The role of animal fats as a carcinogenic factor remains unclear. Although fats are considered to increase the risk of cancer, there is neither compelling evidence from epidemiological studies nor any other indication that a causal relationship exists. This statement does not address the role of fats as energy source or their possible role in the development of obesity.
 Carcinogens and Cocarcinogens in the Food
Carcinogens and Cocarcinogens in the Food
Carcinogens and cocarcinogens occur in our food naturally, but they are also generated during processing, storage, and preservation. Natural ingredients with mutagenic activity include, for example, hydrogen cyanide-containing glycosides in bitter almonds and kernels of stone fruits, and solanine in potatoes (28).
It is still not clear what role additives and residues play in carcinogenesis, but they seem to play a minor role as compared with other factors of nutrition (61).
Alcohol
Alcohol is a risk factor for carcinogenesis in the upper respiratory and digestive tracts, in particular (45). In most epidemiological studies examining this relationship, a direct correlation has been demonstrated-and the risk rises with increasing consumption (26). Alcohol itself does not have a carcinogenic effect, but it acts as a cocarcinogen and thus promotes the development of cancer through various mechanisms. In addition, carcinogenic compounds have been detected in various alcoholic beverages, such as polycyclic aromatic hydrocarbons and nitrosamines (61). Alcohol also influences eating habits, metabolism, and adsorption, thus resulting in a poor supply of protective substances (20).
High-proof alcoholic beverages significantly increase the risk of cancer, more so than beer or wine. A dose-effect relationship has also been observed with tobacco use, which often accompanies chronic alcohol consumption. Both factors multiply each other’s effect (74).
Fats
A diet rich in fat is thought to promote the development of various types of cancer. There are clear indications that the levels and types of fat consumed correlate with the risk of colon cancer, while a connection between fat intake and cancer of the breast, prostate, endometrium, and ovaries is still being debated (97).
Epidemiological and experimental studies indicate that, especially with colon cancer, it is not only the level of total fat intake that plays a decisive role but also the pattern of fatty acids. Foods with a high proportion of saturated fatty acids (SFAs) seem to increase the risk of colon cancer. The same is true for a high intake of omega-6 fatty acids, whereas omega-3 fatty acids-primarily present in fish oil-have a protective effect (1). According to the new reference values for nutrient intake used in the United States (29) and in Germany, Austria, and Switzerland (DACH reference values, 22), consumption of fat should not exceed 30 % of the total energy intake, and the ratio of omega-3 fatty acids to omega-6 fatty acids should be raised to at least 5:1.
Studies examining the influence of the amount of fat intake on the development of breast cancer yielded contradictory results. Experimental findings that fats may promote carcinogenesis have not been confirmed by prospective cohort studies. It is now increasingly debated whether it is really the amount of fat that plays the decisive role, or whether it is actually the total energy content of the diet-and the obesity associated with it- that increases the risk of breast cancer in women (100).
Recent findings indicate a close relationship between the levels of estrogen and insulin circulating in the blood. It has been demonstrated that insulin resistance can raise the level of free estrogen available to cells, either by lowering the level of estrogen-binding globulins in the plasma or by increasing androgen production in the ovaries. Insulin resistance, on the other hand, is closely related to eating habits: a diet rich in fat leads to a higher proportion of free fatty acids in the plasma, thus causing an increase in the fasting glucose level by means of various metabolic steps. Like a diet with a high glycemic index, this leads to hyperinsulinemia—which, in turn, promotes the development of insulin resistance. Based on these mechanisms, a diet rich in fat may be a potential risk factor for breast cancer by contributing to both intra-abdominal accumulation of fat and insulin resistance (20).
Meat
Some meats and meat products contain a high portion of fat. A diet rich in meat therefore often leads to a high intake of energy, fat, and protein. The iron intake associated with the consumption of meat may also play a role as iron promotes the formation of radicals. In addition, a diet rich in meat is often associated with low consumption of fruits and vegetables.
Increased protein intake results in an increased production of urea, which is broken down in the colon to ammonia. Ammonia causes a rise in cellular metabolism, an increased susceptibility of the cells to viral infections, and an enhanced effect of mutagens. Hence, ammonia is generally regarded as a cocarcinogen (88).
The usual methods of food preparation, such as frying and grilling, may give rise to heterocyclic amines and polycyclic aromatic hydrocarbons, which have been shown to be carcinogenic in animal experiments (20).
Nitrosamines
Of over 300 known N-nitroso compounds, about 90 % proved carcinogenic for various organs when tested in animal experiments. Therefore, their role in carcinogenesis in humans has been investigated for many years (26).
Nitrosamines are produced from nitrate and secondary amines through bacterial activity. A major source of nitrate is a diet rich in vegetables. Certain vegetables—such as red beet, spinach, and leaf lettuce—may contain considerable amounts of nitrate, especially when grown in a hot house and when treated heavily with fertilizers. Even drinking water may be rich in nitrate, particularly when nitrate-containing fertilizers get into the water. In meat processing, the nitrate present in pickling solutions is used to redden and cure the meat (19).
Nitrosamines formed in the stomach play a major role in the development of gastric cancer. Their effect is favored by the hypoacidic state of the stomach caused by chronic inflammation. Hypoacidity stimulates bacterial growth, thus speeding up the reduction of nitrate to nitrite. Sodium chloride promotes the development of atrophic gastritis and thus acts as a cocarcinogen. Improved methods of preservation have considerably lowered the risk factors for gastric cancer; it is far less common today that it has been in the past (31).
Mycotoxins
Mycotoxins are produced by molds developing primarily due to improper storage. They are widespread in tropical and subtropical countries, the biggest problem being aflatoxin. Above all, mycotoxins may contaminate peanuts, other types of nuts and seeds, rice, and other grains. Moldy bread should not be eaten because of its aflatoxin content. Just removing the moldy parts is not sufficient because aflatoxin quickly penetrates into deeper layers of the bread without being visible from the surface (81).
Other toxins occur in fruits and vegetables and in products prepared from them. Of special importance is patulin generated in fruits affected by blight (61).
Some mycotoxins proved to be highly carcinogenic in animal experiments and are thought to induce cancer in humans as well (20). There is convincing evidence that they may cause liver cancer.
Polycyclic Aromatic Hydrocarbons (PAHs)
PAHs are generated during incomplete combustion and during flash pasteurization of organic matter. PAHs are widespread because such processes are very common not only in domestic settings but also in the food industry. Traces are also found in unprocessed foods. The indicator compound of PAHs is the highly carcinogenic benzopyrene. It is found in foods derived from both plants and animals, and its content may vary with the location. The main source, however, is cigarette smoke. Benzopyrene is therefore thought to be primarily a carcinogen of the respiratory tract, although it seems to have an effect on the development of gastric cancer and intestinal cancer as well.
In the case of animal products, especially food processing plays a major role—apart from environmental pollution—because benzopyrene is also produced during broiling and smoking. High concentrations of PAHs are generated when meat is barbecued over an open fire, particularly when fat is dripping into the fire (23),(24),(85).
Heterocyclic Aromatic Amines (HAAs)
HAAs are produced when food—mainly meat and fish—is heated at temperatures commonly used in the kitchen. Especially the crust is heavily loaded with HAAs, and the content mainly depends on the temperature and the duration of heating. In fact, the small amounts taken in with food are not regarded as harmless because of their mutagenic and carcinogenic properties. Possibly, the HAA content—rather than the meat itself—is responsible for the well-established correlation between meat consumption and cancer risk. As a preventive measure, it is recommended that meat and fish be prepared by more gentle methods, such as stewing. Unnecessarily high temperatures and long preparation times should be avoided when frying, roasting, or barbecuing. Using the juice from heavily roasted meat for the preparation of gravy is not recommended (39).
Obesity
It has been observed for many years that there is a correlation between a high body mass index (BMI, body weight in kg/square of the height in m) and physical inactivity, on the one hand, and an increased risk of certain types of cancer, on the other. Perhaps this can be explained by elevated levels of insulin and other hormones known as growth factors. Consistently elevated insulin levels, favored by insulin resistance at the cellular level, may increase the rate of cell division and thus raise the risk of mutations (105).
Elevated levels of sex hormones and reduced concentrations of sex hormone-binding globulins have been found to be associated with an increase in body mass. Enzymatic conversion of androgens into estrogens can take place in fatty tissue, which again leads to elevated estrogen levels. Estrogens represent a major factor in the development of mammary and cervical carcinomas. The correlation between obesity and the risk of breast cancer has been observed primarily in postmenopausal women (20). In the case of endometrial carcinoma, studies have demonstrated a twofold to tenfold higher risk associated with postmenopausal obesity. Especially at an advanced age, being obese or gaining weight seems to have unfavorable consequences (26).
 Dietary Components Effective in Prevention
Dietary Components Effective in Prevention
Diet can influence the process of carcinogenesis at various stages: first, by affecting the initial stages of carcinogenesis (primary prevention), by preventing the malignant transformation of precursor cells (secondary prevention), and by preventing the recurrence of the disease following a recovery (tertiary prevention) (51).
No form of a diet can prevent cancer with certainty, and epidemiological evidence of a correlation between diet and cancer is still incomplete; yet it is possible to establish dietary recommendations that might reduce the risk of developing cancer (26).
The positive health benefits of a diet rich in fruits and vegetables are now common knowledge. However, the question of what the underlying mechanisms may be is still the subject of ongoing research. Experimental research, in particular, has shown that there are many biologically plausible explanations for the anticancer properties of plant-derived food. However, the protective effect cannot be traced back to individual ingredients. According to currently available scientific findings, it is rather the food pattern—the selection, preparation, and amount of food—that seems to be essential for influencing the risk of cancer and other chronic diseases. It seems as if the effects associated with individual food ingredients are adding up and thus determine the risk of developing cancer. However, it is still not clear which mechanisms of action are relevant in humans (20).
A great number of substances that occur naturally in foods are regarded as protective factors against the development of cancer. These include antioxidants (e.g., β-carotene, and the vitamins A, C, and E), calcium, selenium, zinc, fiber, secondary plant metabolites, and lactic acid. Also considered are riboflavin and folic acid (61).
When considering individual nutrients in our food, we should be aware of the fact that they always exist in a close relationship with other nutrients. For example, a low intake of fat is correlated with a reduced risk of breast cancer, on the one hand, but associated with a reduced level of fatsoluble vitamins, on the other. We need to gather results that can be properly interpreted. When assessing certain types of diet, it makes sense to define specific indicator substances (markers) that, either alone or integrated, are known to have a preventive effect against the development of cancer. These markers should be detectable in human blood and tissues and be correlated in a quantifiable manner with the respective food items. The following indicator substances are often used:
• in vegetables: β-carotene (as well as vitamin A)
• in vegetable oils: vitamin E
• in fruits, especially citrus fruits: vitamin C.
These markers can be used for interpreting epidemiological studies as well as for analyzing direct interactions in animal experiments and in-vitro assays. A final proof of their isolated mechanisms of action, however, is only possible through targeted dietary intervention studies (6).
Antioxidants
Antioxidants protect DNA and cell membranes from damage caused by oxidation and thus contribute to protecting the organism from mutations and maintaining the cell’s integrity. This protective effect is based on the ability of antioxidants to prevent the formation of free radicals by capturing reactive oxygen compounds, or to make an electron available to existing radicals without becoming a reactive radical itself. The so-called exogenous or nonenzymatic antioxidants (vitamins C and E, β-carotene, and some other carotenoids) together with the endogenous or enzymatic antioxidants (superoxide dismutase, glutathione peroxidase, catalase, and others) form an effective protective shield against aggressive oxygen compounds (53).
The balance between free radicals and antioxidants can be disturbed by a number of exogenous and endogenous processes; as a result, the protective antioxidant potential may soon become exhausted. Exogenous tumor initiators include smoke, ionizing radiation, ultraviolet light, ozone, as well as dietary factors and deficits. In addition, highly reactive oxygen compounds are generated endogenously as by-products of the cytochrome system and also in activated leukocytes and macrophages (26).
Oxidative processes contribute to the production of free radicals or other reactive oxygen species. Oxygen radicals can modify deoxyribose or DNA bases and thereby trigger mutations. In addition, they can cause severe changes in the biological activity of proteins. In addition, lipids, especially the highly unsaturated fatty acids in membranes, are often subjected to oxidative damages (63). Free radicals are involved at every stage of carcinogenesis (55).
Based on their different properties of solubility, antioxidants are active either in the hydrophilic or lipophilic phase. Selenium is an essential component of the two endogenous antioxidant enzyme systems, glutathione peroxidase (GSH-Px) and phospholipid-hydroperoxide-glutathione peroxidase (PH-GSH-Px), and zinc plays an important role in superoxide dismutase. The hydrophilic vitamin C and GSH-Px are active in the aqueous environment of the cell, whereas lipophilic anti-oxidants-such as vitamin E, β-carotene, and PH-GSH-Px-are active within the membranes. The various protective systems act synergistically; they can regenerate each other but cannot replace one another (26).
In addition to essential nutrients that possess antioxidant activity, secondary plant metabolites with proved antioxidant activities are becoming the focal point of research (26).
In terms of primary prevention, the statistical analysis of epidemiological data has led us to the conclusion that regular and ample consumption of vegetables, fruits, and whole-grain products can reduce the risk of cancer (17). This can be explained primarily by the intake of natural antioxidants that are associated with this type of diet, for example, β-carotene, vitamin C, vitamin E, and selenium. However, this does not prove a causal relationship between individual antioxidants and the protection from cancer (22).
Table 8.2 Reference values for plasma levels considered optimal for cancer prevention (22)
| Vitamin E | >30 μmol/L |
| Vitamin C | >50 μmol/L |
| β-Carotene | >0.4 μmol/L |
| Selenium | > 50 μg/L |
Based on epidemiological data, plasma concentrations of antioxidants have been established as reference values for the primary prevention of cancer in healthy adults. These values have been derived from the results of prospective studies and case studies and also from comparisons between various countries (Table 8.2).
To reach the established plasma concentrations, a daily intake of 75–150 mg of vitamin C, 1530 mg of vitamin E (α-tocopherol equivalents), and 2–4 mg of β-carotene in the diet has been recommended at a consensus conference in 1995 (4). However, these recommendations are only valid for healthy persons who are not exposed to special oxidative stress. According to the VERA Study analyzing the relationship between nutrition and risk factors in Germany, the reference values for plasma concentrations can be achieved by just eating a normal diet (43). It should be taken into account, however, that the target concentrations may not be reached in some individuals despite a wholesome diet. Underlying causes may include a variable relationship between intake and plasma concentrations as well as increased requirements due to oxidative stress. Upon consultation with a physician, additional intake of antioxidants in the form of dietary supplements may be indicated. Under no circumstances, however, should this be used to offset an unhealthy diet and/or lifestyle (22). Current knowledge on the mutual supplementation and enhancement of antioxidant substances suggests that eating a wholesome diet should always be preferred to substituting individual active substances by dietary supplements.
To ensure the intake of antioxidant vitamins in the amounts required for prevention, the National Academy of Science has recommended that Americans eat fruits and vegetables five times a day (18). The German Society of Nutrition has developed a similar concept (“five a day”) to promote the intake of fruits and vegetables. The major arguments for the intake of vitamins through an appropriate diet rather than through dietary supplements are:
• Consuming lots of fruits and vegetables makes it easier to follow the recommendations for a healthy diet, especially with regard to a reduced intake of energy, fat, and sodium chloride.
• Consuming lots of fruits and vegetables increases the intake of fiber, especially of water-soluble fiber, which is broken down by bacteria in the colon. The short-chain fatty acids generated hereby inhibit the development of colon cancer.
• Fruits and vegetables contain also other carotenoids, such as canthaxanthin, lutein, or lycopene, which also possess anticarcinogenic properties and promote cell communication. In addition, these foods are rich in folic acid, which is thought to reduce the risk of cancer further (34).
• Secondary plant metabolites contained in fruits and vegetables have not yet been sufficiently studied, but they most likely possess anticarcinogenic properties (53).
Clinical Studies
During recent years, several intervention studies involving antioxidants have been carried out, and they yielded contradictory results. Basically, the studies have raised the question of whether the often-confirmed negative correlation between the serum concentration of β-carotene and the risk of bronchial carcinoma is indeed causally connected. High concentrations of β-carotene in the serum seem to be rather an indicator of a diet rich in fruits and vegetables—a diet that contributes to cancer prevention through a multitude of protective substances. Other studies also speak against the use of supplements and for a balanced diet with a high proportion of fruits and vegetables in order to prevent cancer (26),(15).
• A positive result was obtained in a cancer prevention study carried out in China (Province of Linxian), a region known for its above—average incidence of gastric and esophageal carcinomas and insufficient or borderline intake of macro-nutrients and micronutrients. Within five years, the combined supplementation of β-carotene (15 mg/day), vitamin E (30 mg/day), and selenium (50 μg/day) resulted in a significant reduction in cancer mortality by 13%. The mortality from stomach cancer even dropped by 21 % as compared with the control group (18). The fact that also the total mortality dropped by 9% and the mortality from cardiovascular diseases by 10%, showed that the study was dealing with a population carrying a high risk due to poor living conditions (53).
• In the Physicians Health Study (PHS), which was completed in 1995 and involved 22 000 healthy, well-nourished American physicians, the risk of cancer was not affected by supplementation with β-carotene. Over a period of 12 years, the study examined whether the intake of 50 mg of β-carotene every second day would reduce the risk of cancer, as compared with a placebo. There was no evidence for a protective effect of β-carotene. There was no difference in the frequency of malignant tumors between the group receiving β-carotene and the placebo group (41).
• The Finnish ATBC Lung Cancer Prevention Study yielded a different result. It was carried out with long-time heavy smokers who took vitamin E (50 mg/day) or β-carotene (20 mg/day) as a supplement. Contrary to expectations, there was no change in the incidence of lung cancer associated with vitamin E, and the death rate increased by 18 % with β-carotene (40). Largely due to high media coverage in the lay press, this negative outcome caused a feeling of unease in the population with respect to foods rich in anti-oxidants. After detailed analysis, however, the negative result turned out to be due to errors in the design of the study (53). One critical aspect was that one could hardly expect to find a preventive effect in the study’s participants after long-time exposure to cigarette smoke. Most likely, the process of tumor development had already been initiated in a large number of participants. At this stage of carcinogenesis, β-carotene can no longer have an effect (36, 26).
• In the CARET Study, smokers and workers exposed to asbestos took β-carotene and vitamin A in order to reduce the risk of bronchial carcinoma. However, the study was terminated prematurely when it was noticed that supplementation was associated with an increased incidence of lung cancer (73).
The results of the Finnish ATBC study and of the two American studies (CARET and PHS) give cause for warning against an uncontrolled intake of pharmacological doses of β-carotene—this is true particularly for groups with an increased risk.
Vitamin A and β-carotene
Vitamin A is taken up as a provitamin (carotenoids) from vegetable sources or in the form of its ester from animal sources. It regulates the expression of many factors—such as growth hormone receptors, oncogenes, and interleukins—which play a special role in the growth and differentiation of cells and tissues (6).
β-carotene is the main representative of a large family of carotenoids, some of which can be converted into vitamin A by our body. However, β-carotene is not only a provitamin but has an antioxidant effect on its own and should be regarded as being essential to humans (6). β-Carotene is able to capture singlet oxygen generated primarily through UV irradiation. The mechanisms discussed include its function as a physical filter (protection from light), quenching properties (emission of energy as heat), and incorporation as a membrane component (replacement of oxidation-sensitive parts). Apart from this, β-carotene is also effective as a chain-interrupting antioxidant by forming stable radicals, particularly at a low partial pressure of oxygen (6). In animal experiments, β-carotene increases the activity of T and B cells and enhances the cellular immune response (20).
• In vitro, retinoic acid (vitamin A acid) inhibits the proliferation of neoplastic cells while promoting their differentiation into normal cells. It has been clearly demonstrated in animal experiments that this vitamin controls, in particular, the differentiation of respiratory mucosa and skin (91). Deficiency in vitamin A typically leads to disturbed differentiation of rapidly growing epithelia, especially of mucosal epithelia (6).
• Numerous epidemiological studies have shown that persons who eat a lot of fruits and vegetables rich in carotenoids have a lower risk of developing certain types of cancer (e. g., carcinomas of the lung, stomach, esophagus, cervix) (22). An inverse relationship was found, in particular, between the incidence of bronchial carcinoma and the intake of β-carotene in the form of fruits and vegetables (102). Lower blood levels of β-carotene carry a higher risk of bronchial carcinoma, in particular (25). In 1991, these findings have prompted the German Society for Nutrition (DGE) to establish a recommended daily intake (RDI) of 2 mg of β-carotene. At the Hohenheim Consensus Conference in 1995, the desirable intake was set to 2–4 mg/day, in order to achieve plasma levels that are considered optimal in preventive medicine. According to the National Consumption Study (NCS) and the VERA Study, these values are generally reached in the average German population. Nonetheless, dietary supplements have become wide spread.
• Requirement. Based on the unfavorable findings of the large intervention studies (ATBC, CARET) involving synthetic β-carotene, the World Health Organization (WHO)-founded International Agency for Research on Cancer advised in a press release on 12 January 1998, against the use of β-carotene and other carotenoids for tumor prevention. The German Federal Institute for Health Consumer Protection and Veterinary Medicine (BgVV) followed suit by issuing an urgent warning against the uncontrolled intake of β-carotene through dietary supplements and enriched foods. A recommendation for the maximal amount of synthetic β-carotene is in effect since 31 January 2001. According to the BgVV, the daily intake should not exceed 2 mg of synthetic β-carotene because a daily intake of 20 mg/day can cause health problems in heavy smokers and other risk groups (32).
• Sources β-carotene is only found in fruits and vegetables, and the amount varies widely depending on variety, degree of ripeness, and the season. Bioavailability depends to a large extent on how the food is prepared. Only about 1 % of β-carotene can be absorbed from raw carrots; mashed carrots or carrot juice can provide much more. The same is true for lycopene from tomatoes.
Vitamin C (Ascorbic Acid)
L-Ascorbic acid reduces water-soluble peroxide radicals as well as other reactive prooxidants and is therefore thought to be an effective antioxidant. It captures radicals in the aqueous phase before they can induce lipid peroxidation. Like vitamin E, vitamin C thus inhibits the peroxidation of cell membranes. It is also capable of regenerating both vitamin E and glutathione. Vitamin C inhibits the formation of nitrosamines both in foods and in the intestinal tract (7). In addition, microsomal hydroxylation reactions in the liver are involved in the metabolism and inactivation of drugs and toxic metabolites (6). The mechanism of some activities—such as the regulation of gene transcription and protein translation—and the significance of accumulation of ascorbate in various immunocompetent cells have not yet been elucidated (22).
Several studies have revealed an inverse relationship between the estimated vitamin C intake and the development of various types of cancer, such as gastric, esophageal, and laryngeal carcinomas (76).
In the 12-year follow-up of the Basel Study, an inverse relationship was found between the consumption of vitamin C-rich foods and the development of stomach cancer. In the 17-year follow-up, however, this effect was no longer statistically significant (26).
A statistically significant inverse relationship has also been observed between vitamin C intake with the diet and breast cancer (46).
• In older persons with low plasma levels of vitamin C, the risk of stomach cancer and cancer of the gastrointestinal tract is increased (96).
• There have also been studies in which no correlation was observed between vitamin C intake and various types of cancer, especially stomach cancer and cancer of the gastrointestinal tract (27).
• Evaluation of all known epidemiological studies up to 1998 revealed that a reduction in the risk of chronic diseases—especially with respect to the morbidity and mortality of cancer and cardiovascular diseases in nonsmokers—is best achieved with plasma levels of more than 50 μmol/L and a daily intake of 90–100 mg of vitamin C (17). Intake of 100 mg ensured optimal saturation of immunocompetent cells. At higher dosages, the renal threshold set in and increased dramatically after intake of more than 200 mg. At the same time, reabsorption of ascorbic acid became less effective.
• Requirement Vitamin C requirement is increased in times of physical stress and with certain diseases, such as diabetes or infections. Based on current knowledge, it is not yet possible to provide a precise figure for these increased needs. Because smoking decreases the absorption and increases the consumption of vitamin C, it is recommended that heavy smokers take 150 mg/day (22).
In 2001, the research of pharmacologists in Philadelphia caused quite a stir when the results were published in the scientific magazine Science (59). According to this study, vitamin C—which acts as an antioxidant within the cells and neutralizes free radicals—may promote the in-vitro production of DNA-damaging compounds. In the experiment, vitamin C and lipid hydroperoxide were combined in a test tube. After two hours, it was observed that so-called genotoxins, which are known to damage DNA, had formed—one of the prerequisites of cell degeneration and, possibly, carcinogenesis. According to the researchers, these findings might explain why vitamin C proved less effective in the fight against cancer than had been expected. However, it would be wrong to conclude from this study that vitamin C would cause cancer and would not contribute to a healthy diet. The results of experiments in vitro cannot easily be applied to the human body. In addition, the notion that vitamin C is essential for human health has been confirmed by other studies.
Vitamin E (Tocopherols)
Numerous retrospective and prospective studies have established a relationship between plasma levels of vitamin E and the risk of cancer in humans. However, the results have not been unambiguously confirmed (80). Other studies have demonstrated that the administration of vitamin E in nontoxic doses has been successful in preventing colon carcinoma and cancer of the mouth and throat as well as esophageal carcinoma (6). Different mechanisms of action are discussed, including the effects of antioxidants in connection with increased cellular immunity. Whereas β-carotene can hardly be discussed separately from foods, this is possible with vitamin E. There is evidence that vitamin E is active also in isolated (synthetic) form (6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree