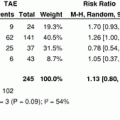
Stage
AJCC- clinical staging
AJCC-staging
I
81 (51 %)
60 (38 %)
II
57 (36 %)
49 (31 %)
III
7 (4 %)
9 (6 %)
IV
0
1 (0.6 %)
No lesions
14 (9 %)
12 (7 %) a
Complete tumor necrosis
28 (17 %)
Table 2
Univariate and multivariate analysis of prognostic factors in LT for HCC in 159 patients
Univariate | Multivariate analysis | |
|---|---|---|
Sex Age B or C cirrhosis Waiting list time ≥6 months Natural-MELD Score ≥15 Child-Pugh A-B versus C ≥3 nodules Nodule ≥5 cm Milan in UCSF in Bridging therapies ≥2 bridging therapies Downstaging AFP level ≥100 ng/ml AFP level ≥400 ng/ml IVC replacement Veno-venous by-pass Cell-saver use 100 % tumor necrosis on liver specimen Tumoral capsule effraction Microvascular invasion Satellite nodules Cyclosporine monotherapy regimen Tacrolimus monotherapy regimen Corticoresistant rejection treated antibodies | NS NS NS NS NS NS NS P = 0.0187 P = 0.0008 P = 0.0018 P = 0.0289 NS NS P = 0.0024 P = 0.0004 NS P = 0.0297 NS NS NS P = 0.0441 NS NS NS P = 0.0011 | NS NS NS NS NS NS P = 0.029 NS P = 0.003 NS NS NS NS P = 0.006 NS NS NS NS NS NS NS NS NS NS P = 0.0036 |
After 48 months (range 3–190) follow-up, actuarial overall survival (OS) and disease-free survival (DFS) at 1, 3, 5, 10 years were 88, 76, 66, 57 % and 86, 75, 66, 57 %, respectively (Fig. 1). Fifty-two (32.7 %) patients died during follow-up; 20 (12 %) patients died of tumor recurrence at a mean of 510 days (range 90–2593). The 5-year DFS rates of patients inside versus outside MC were 74 and 47 %. The 5-year DFS rates of patients inside versus outside UCSF criteria were 72 and 41 % (Fig. 2). The results markedly improved after 1992 when the approach to these patients was systematized (Fig. 3).
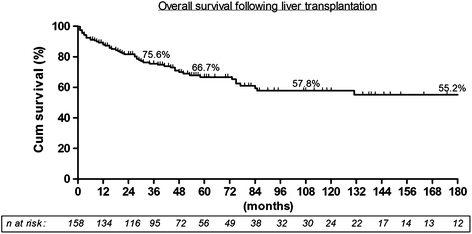
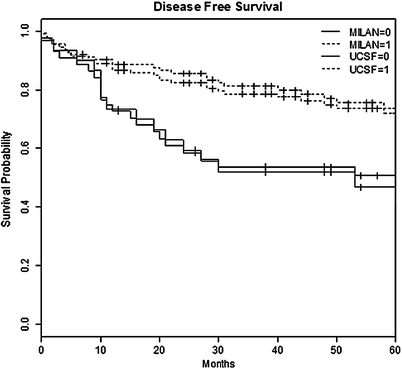
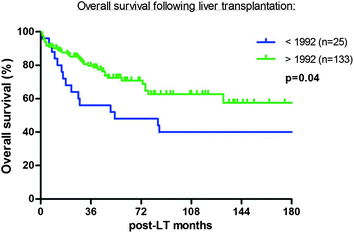

Fig. 1
Overall long-term survival after LT for HCC

Fig. 2
Actuarial survival after LT for HCC in relation to Milan In/out and UCSF In/out criteria

Fig. 3
Improvement of results after implementation of a systematized approach to LT including pretransplant locoregional treatments
Neoadjuvant LRT did not influence outcome of MC in patients (89 % without LRT vs. 95 % DFS survival with LRT). LRT was associated with improved 5-year disease free survival in patients outside MC (66 vs. 45 %, p 0.03). At pathological examination of surgical specimen, partial (50 % or more) or complete tumor necrosis was detected in 45 and 17 % of patients who received preOLT LRT, respectively. Complete tumor necrosis after LRT was associated with improved 5-year DFS (71 vs. 62 %, p 0.05).
In univariate analysis, tumor diameter >5 cm, MC and UCSF in, LRT, AFP level over 100 ng/ml, use of VVB, microvascular invasion and use of mono- or polyclonal antibodies in the treatment of (corticosteroid resistant) rejection were associated with outcome. In the multivariate analysis, number of nodules less than four (this independent of tumor size) (72 vs. 59 %, p 0.02), MC in (74 vs. 47 %, p 0.003), AFP level of more than 100 ng/ml (73 vs. 39 %, p 0.06), and use of anti-lymphocytic antibodies (70 vs. 36 %, p 0.0036) were identified as independent predictors of DFS after OLT for HCC (Table 2).
4 Particular Features in Relation to OLT and HCC
4.1 Technical Considerations
4.1.1 Partial Liver Resection: Bridge to LT or Salvage LT
As shown by many very large experiences from East and West, partial liver resection plays an important role in the treatment of HCC in cirrhosis [27, 65, 83]. Unfortunately PLR can only be offered to about 5–10 % of cirrhotic patients due to their insufficient hepatic functional reserve [8]. In patients with resectable HCC and preserved liver function, the place of each treatment modality is still debated, resection can be used as an alternative to OLT, resection can be followed by insertion on the waiting list (‘bridge’ resection to OLT), resection can be followed by LT only in case of recurrence (‘salvage LT’), and finally OLT as first treatment option; this latter attitude is based on the very high recurrence rates of PLR [50].
The Barcelona group showed that LRT is justified when the waiting time is superior to one year; when waiting time is shorter local tumor destruction is more advantageous than PLR [46]. Besides economic arguments, previous resection is likely to increase technical difficulty and morbidity-mortality of OLT. However, the clinical impact of previous resection on early and long-term postoperative outcome of OLT for HCC is controversial. The Paul Brousse group reported higher postoperative mortality rate after OLT in patients who had previously undergone liver resection [2]. In contrast, the Beaujon group and Creteil group did not show any impact on postoperative results of OLT in resected patients. In addition, in these latter series, resection was likely to improve selection for OLT [5, 11]. A laparoscopic approach to resection may be a safe and valuable option as postoperative adhesions seem to be less developed [36]. Recent studies comparing resection as a bridge to OLT, to primary OLT, suggest that primary OLT is associated with slight but significantly improved outcome (24 % median 5 year DFS increase, range 2–40 %) [12, 65]. In a large US multicenter comparative study including patients outside MC, 3-year survival was similar in patients undergoing resection as a bridge to OLT and patients who had primary OLT, however, cancer-related mortality rate was higher in patients who underwent resection whereas non-cancer mortality rate including viral infection-related death was higher in patients who had primary OLT [9]. Cherqui et al. recently reported good outcome following salvage OLT. In this series 78 % of patients who developed recurrence after resection could be candidate to OLT and 44 % underwent salvage OLT. In intent to treat analysis, 5-year OS and DFS rate was 72 and 44 % [11]. Despite the relatively good results of salvage OLT, a subset of patients could not ultimately undergo OLT. In addition, in a recent series, time to recurrence less than 1 year was associated with poor outcome after salvage OLT [70]. Therefore, our policy at Saint Luc Hospital is to propose primary OLT to decrease as much as possible the risk of dropout considering that OLT remains the best treatment option in patients inside transplantation criteria. When waiting time exceeds 6 months or 1 year, non-surgical LRT, which provide acceptable outcome at three years, are preferred options.
4.2 Consequences of Neoadjuvant Locoregional Therapy
Clinical impact of previous LRT on postoperative outcome after OLT is less marked than impact of previous resection. However, repeated use of TACE can cause severe damage to the arterial wall due to endothelitis, peri-adventitial thickening, and even thrombosis due to the catheterization [47] complicating vascular reconstruction. Extensive dissection of the hepatic artery (even up to the celiac trunk) can be needed in order to perform arterial anastomosis in safe tissue. In case of major damages to the recipient hepatic artery, anastomosis of the donor artery to the reversed splenic artery can be performed [20].
4.3 Inferior Vena Cava Preserving Hepatectomy
The standard technique of OLT included the removal of retrohepatic IVC with the cirrhotic liver. Progresses in surgical technique and technical skills have led to the wide application of the IVC preserving total hepatectomy [39]. This approach is still questioned in case of HCC especially when the tumor is located in the right posterior segments or segment I close to the IVC. The probability of tumor involvement of the IVC has been studied extensively by the Tokyo group [25, 53]. IVC wall invasion is very unlikely in the absence of clinical symptoms or signs such as edema of lower limbs. In our series, patients which underwent IVC removal showed poorer outcomes compared to those in patient with IVC preservation in overall survival (p < 0.04).
4.4 Intraoperative Blood Salvage and Veno-Venous Bypass
The use of intraoperative blood salvage (IOBS) during OLT for HCC has long been controversial. Several studies have demonstrated the safety of IOBS in OLT for HCC [45, 59]. IOBS does not affect HCC recipients’ oncologic results. The use of a leukocyte-depletion filter, which removes possible tumor cells and also bacteria, enhances the safety of the blood handling. IOBS should not be used in very peripheral tumors at risk of rupture during liver dissection. Veno-venous bypass is widely used for OLT. The introduction IVC sparing technique has raised the question of the utility of VVB in OLT. VVB does not affect oncologic outcome of patients undergoing OLT for HCC [4, 13]. Indication of VVB during OLT more likely relies on technical condition during surgery rather than the type of indication OLT.
4.5 Hepatocellular Carcinoma Allograft Recurrence
The markedly improved long-term results obtained after OLT for HCC also led to a more aggressive medical as well as surgical approach to the intra- and even extrahepatic tumor recurrence [15, 23, 35, 65, 100]. Surgical resection of the recurrence is associated with improved survival despite a high risk of further new recurrence [51, 84, 92]. In patients with extrahepatic or multifocal recurrence, the use of sorafenib or a change in immunosuppressive treatment based on rapamycin can be used [31, 32, 87, 101]. These two treatment modalities can be combined with a synergic antitumoral effect.
5 Particular Medical Features
5.1 HCC in a Non-Cirrhotic, Non-Fibrotic Liver
The standard treatment approach in patients with resectable HCC in non-cirrhotic liver is resection. Only few studies including a small number of patients have evaluated the interest of OLT in HCC developing in non-cirrhotic liver (NC-HCC) is small [26, 58]. Five-year OS and DFS rates following liver resection for NC-HCC range from 25 to 81 % and from 24 to 59 %, respectively [91]. The reported incidence of tumor recurrence ranges from 30 to 73 %. This high recurrence rate after PLR and the very few reports of successful outcome after LT done for intrahepatic recurrence after partial resection in such patients may indicate that NC-HCC represents an underused indication for OLT. The analysis of 27 reported, well-documented, NC-HCC liver recipients (including also FL-HCC) and of 62 patients, collected by the European Registry for Liver transplantation (ELTR), transplanted for unresectable NC-HCC (also including fibrolamellar-HCC) shows that OLT may also be indicated in selected patients with NC-HCC [41]. The best indications may be cases where R0 liver resection cannot be guaranteed. In the ELTR study, 5 years overall survival was 49 %. Patients without risk factors for tumor recurrence such as absence of vascular and/or lymph node involvement had a five years survival of 59 %. Only 4.8 % of patients fulfilled MC. In line with the data obtained after liver resection, factors predicting outcome after OLT for NC-HCC were different from those identified in patients with HCC developing in chronic liver disease. Only major vascular invasion and lymph node invasion were found as independent predictor of outcome in patients undergoing OLT in NC-HCC. Salvage OLT has been proposed in patients with liver recurrence after resection of NC-HCC. Outcomes are similar to those after primary OLT with 58 % 5-year OS and 48 % 5-year DFS rates. Macrovascular invasion and a time to recurrence of less than 12 months were associated with outcome. Improved pathological assessment of the resected tumor and of the non-tumor liver tissue may be helpful in identifying patients at high risk of tumor recurrence after PLR and OLT [41, 57, 58].
5.2 Immunosuppression
OLT is followed by a life-long medical treatment compromising the immunological status of the patient that may interfere with the risk of tumor progression in HCC patients. We and others have reported that immunosuppression using anti-lymphocytic antibodies in particular could negatively affect outcome of HCC patients who underwent OLT [16]. Additionally, the net IS status of the patient defined by calcineurin inhibitor serum level has been associated with outcome in cancer patients [95]. Tailoring IS therapy to patients’ oncologic status is likely to play an important role when extending the inclusion criteria and minimizing IS should be regarded as an objective in patients with advanced tumor stages [40, 66] The use of m-Tor inhibitors as immunosuppressive therapy in patients transplanted for HCC may represent a valuable alternative since these molecules have also antiangiogenic properties [44, 74, 75, 89]. In addition, several studies have shown that that m-Tor inhibitors can be safely associated with sorafenib as an adjuvant treatment in recipients at high risk for recurrence [21, 68, 86].
6 Conclusion
OLT is the best treatment option in patients with HCC and chronic liver disease. Unfortunately, this treatment modality can only be proposed to minority of HCC patients. To date, a lot of questions remain especially regarding the extension of transplantation criteria. Different treatment modalities including resection, LRT, and medical therapy can be used to control tumor progression and render patients initially deemed outside transplantation criteria potential transplant candidates. These treatment modalities should not be regarded as competitive treatment options and the choice for the best treatment strategy should be adapted to patient liver function and tumor extent without excluding any treatment options.
References
1.
A simplified American Joint Committee on Cancer (AJCC) (2002) TNM staging system for hepatocellular carcinoma (HCC) 6th edn
2.
Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H (2004) Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg 240(5):925–928
3.
Adler M, De Pauw F, Vereerstraeten P, Fancello A, Lerut J, Starkel P, VanVlierberghe H, Troisi R, Donckier V, Detry O, Delwaide J, Michielsen P, Chapelle T, Pirenne J, Nevens F (2008) Outcome of patients with hepatocellular carcinoma listed for liver transplantation within the Eurotransplant allocation system. Liver Transpl 14(4):526–533PubMedCrossRef
4.
5.
6.
7.
8.
9.
Canter RJ, Patel SA, Kennedy T, Angelica DMI, Jarnagin WR, Fong Y, Blumgart LH, Freeman RB, Dematteo RP, Abt PL (2011) Comparative analysis of outcome in patients with hepatocellular carcinoma exceeding the Milan criteria treated with liver transplantation hepatectomy versus partial hepatectomy. Am J Clin Oncol 34(5):466–471PubMedCrossRef
10.
11.