Fig. 1
Incidence and mortality of different malignancies GLOBOCAN 2012 Ferlay J IARC Internet based
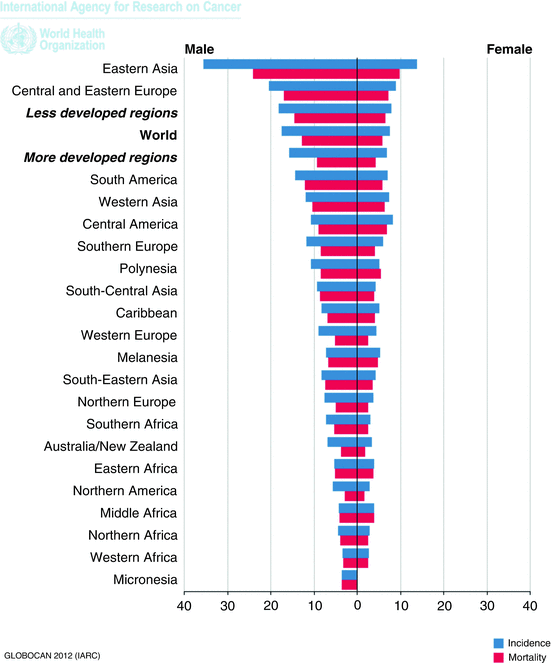
Fig. 2
Incidence and mortality of gastric cancer per region. GLOBOCAN 2012 Ferlay J IARC Internet based
Stomach cancer is the third leading cause of cancer death in both sexes worldwide (723,000 deaths, 8.8 % of the total) third to lung and liver malignancies. The highest estimated mortality rates are in Eastern Asia (24 per 100,000 in men, 9.8 per 100,000 in women), and the lowest are in Northern America (2.8 and 1.5, respectively) [2]. High mortality rates are also present in both sexes in Central and Eastern Europe, and in Central and South America.
Gender-specific incidence and mortality are double in men compared to women.
GLOBOCAN 2012 shows the trend in both incidence and mortality since 1975 and the tendency is towards a marked decline in nearly all populations, irrespective of whether the population is at high risk (Japanese males) or low risk (US white females). This can be attributed to changes in food handling, refrigeration, abundance of fresh fruit and vegetables, the decrease in the use of tobacco and salt, but above all the decreased exposure to a ubiquitous risk factor, H. pylori.
Risk Factors/Pathogenesis
Helicobacter pylori
H. pylori is gram-negative bacterium that colonizes the stomach, and even though the majority of infections are asymptomatic, H. pylori is associated with peptic ulcer disease, chronic gastritis, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric adenocarcinoma. It is thought that H. pylori was once ubiquitous to mankind but its prevalence is declining in successive generations, and is now very rare in Western Europe, North America, Australia and Japan [3]. The decreased incidence and prevalence in these developed countries can be explained by the fact that infection risk is associated with overcrowding, poor sanitation and low socioeconomic status [4, 5]. The decreased prevalence of H. pylori is matched to the decline in incidence and mortality of gastric cancer.
The International Agency for Research on Cancer (IARC) classified infection with H. pylori as carcinogenic in 1994 based on its association to gastric cancer and MALT [6]. This conclusion was confirmed by IARC in 2009 stating that H. pylori causes non-cardiac gastric cancer due to the confinement of H. pylori to the distal part of the stomach [7]. The best estimate of relative risk for H. pylori and gastric cancer comes from the Helicobacter and Cancer Collaborative Group which performed a pooled analysis of 12 prospective studies, including 762 cases of non-cardiac cancer and 2250 controls. The odds ratio for H. pylori was 2.97 [8]. The same study included 274 cases of cardia gastric cancer and 827 controls with an odds ratio of 0.99 for H. pylori infection.
H. pylori is genomically highly diverse and this diversity may contribute to the clinical outcome of the infection. Several genetic factors associated with H. pylori colonization and virulence (cagA, acA) have been identified. The genetic marker that has attracted the most attention in epidemiologic studies is the presence of the cag pathogenicity island, a DNA sequence of 40 kbp that is present in 70 % of H. pylori strains in Europe and North America, but is ubiquitous in Asia and most of Africa. CagA-positive strains are associated with higher risk of gastric cancer than CagA negative strains. A meta-analysis of 16 cohort and case–control studies including 778 cases of non-cardia gastric cancer and 1409 matched controls found an elevated risk of CagA-positive H. pylori infections, with an OR of 2.01 for CagA-positivity among all H. pylori-infected individuals [8].
Socioeconomic Status
Less developed countries exhibit higher incidence and mortality of gastric cancer, presumably secondary to untreated prevalent H. pylori infection. But even within a given country or population, non-cardia cancer is seen much more commonly in individuals with surrogates associated with a lower socioeconomic status (lower education, number of siblings, crowding) [9, 10].
Tobacco and Alcohol Abuse
A meta-analysis performed by Ladeiras-Lopes et al. showed that the summary risk estimate of gastric cancer was 1.62 in male smokers and 1.2 in female smokers compared to nonsmokers [11]. The risk for cancer is known to increase significantly with increasing numbers of cigarettes per day, pack-years, or duration of smoking [12]. Although alcohol is considered a risk factor for gastric cancer, prospective studies have failed to show an increased relative risk compared to the control group [13].
Body Mass Index and Physical Activity
A meta-analysis by Yang et al. has shown increased risk for cardia gastric cancer in patients with a BMI above 30 with a relative risk estimate of 2.6 [14]. With non-cardia gastric cancers, however, cohort studies have failed to show an increased risk in obese patients [15]. Regular physical activity is known to be associated with decreased risk of gastric cancer, and two prospective studies, one from Norway and one from the USA studying physical activity and gastric and esophageal cancer have shown a protective effect [16, 17].
Pathogenesis
There are two models of carcinogenesis in gastric cancer, the Correa pathway for the intestinal type [18, 19] and the Carneiro pathway for the diffuse type of adenocarcinoma [20]. Figure 3 summarizes the Correa pathway, a multistep process with multiple genetic and epigenetic alterations that may take years to decades to develop. Chronic H. pylori gastritis leads to genomic instability (dysfunction of the DNA mismatch repair system) and through DNA methylation leads to atrophic gastritis, defined as the loss of normal glandular epithelium, and the lost glandular epithelium is replaced by intestinal epithelium, leading to metaplasia. There are two main types of metaplastic cells. The first are spasmolytic polypeptide-expressing metaplasia (SPEM) cells, found in 68 % of patients with H. pylori infection and associated with 90 % of gastric cancers. Therefore SPEM may be a step in neoplastic progression. The second type of metaplasia is intestinal metaplasia, which may arise in the background of SPEM or in the native gastric epithelium. Intestinal metaplasia is also associated with increased risk of gastric cancer as well as gastric ulcers and chronic gastritis. Mutations in tumor suppressor genes (APC, TP53) lead to dysplasia, which then is followed by loss of heterozygosity at the DCC (deleted in colon cancer) locus and APC (adenomatous polyposis coli) gene mutation, leading to intestinal type carcinoma.
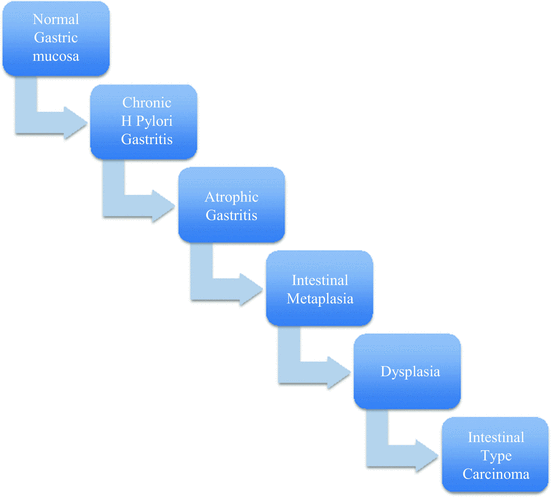

Fig. 3
Correra model of intestinal type gastric cancer carcinogenesis
The Carneiro pathway is based on germline mutations in the E-cadherin gene CDH1. Mutations of CDH1 gene are responsible for 30–40 % of hereditary diffuse gastric cancer syndrome. The second hit occurs in most cases by epigenetic silencing of CDH1 promoter by methylation, leading to signet ring carcinoma in situ and ultimately to invasive signet ring carcinoma [20].
Classification
There are different classification schemes for gastric cancer, based on anatomic location within the stomach, histological subtype, as well as degree of invasion.
Anatomic Classification
Based on the location of the carcinoma, gastric cancer can be divided into cardia (proximal stomach) and distal (non-cardia). The incidence of distal tumors has decreased in recent decades; in contrast, the occurrence of proximal tumors has increased, especially in industrialized countries, apparently related to gastroesophageal reflux disease. Adenocarcinomas of the cardia display a far more aggressive behavior, invading the gastric and esophageal walls and metastasizing to local lymph nodes; the 5-year survival rate is less than 15 % in the USA.
Of clinical interest is the distinction between distal esophageal and proximal gastric cancers and the management of those lesions. The Siewert classification divides the lesions into three types:
Type I: adenocarcinoma of the distal part esophagus (tumor center located between 1 and 5 cm above the anatomic gastroesophageal junction (GEJ ) ).
Type II: adenocarcinoma of the true gastric cardia (within 1 cm above and 2 cm below the GEJ).
Type III: adenocarcinoma of the subcardial stomach (2–5 cm below the GEJ) [21].
Siewert proposed esophagectomy as the procedure of choice for Type I lesions and total gastrectomy with extension into the esophagus for Type II and III lesions [22]. The American Joint Commission on Cancer Classification (AJCC) considers tumors of the gastroesophageal junction including the esophagus and the proximal 5 cm of the stomach as esophageal carcinomas and recommends the clinical management of these tumors follow the guidelines for esophageal cancer [23]. A lesion with its center within 5 cm of the GEJ but without extension into the esophagus, and a lesion with its center greater than 5 cm away from the GEJ are staged using gastric carcinoma criteria [24].
Degree of Invasion
Based on the degree of invasion gastric cancer can be classified as early or advanced. Early cancers are those limited to the mucosa and submucosa irrespective of lymph node involvement. The 5-year survival rate is 85–100 % for early gastric cancers. Advanced gastric cancers are those invading beyond the submucosa and are divided according to the Borrmann classification to (1) polypoid, (2) ulcerated, (3) ulcerated infiltrating, and (4) diffusely infiltrating (also known as linitis plastica ). The 5-year survival for advanced cancers is 5–20 %.
Histologic Classification
The Lauren classification is the most commonly used histologic classification system, and it divides the tumors into two types: intestinal and diffuse.
Intestinal type is so named due to the glandular forming neoplastic epithelium, with cellular cohesion. Intestinal is the most common histologic type, found in high-incidence populations.
Diffuse-type gastric cancer histologically is notable for a lack of cellular cohesion, with independent cellular islands of tissue invasion. Signet ring cells are classified as diffuse type [25].
Presentation
The most common symptoms at initial diagnosis of gastric cancer are weight loss (62 %) and persistent abdominal pain (52 %). Weight loss is often secondary to insufficient caloric intake that may be attributable to anorexia, nausea, early satiety, and dysphagia. Dysphagia is present in 26 % of patients and is a common symptom in cancers arising in the proximal stomach. Abdominal pain tends to be epigastric, vague and mild in early disease but more severe and constant as disease progresses. Nausea (34 %) is present in patients with linitis plastica with poor gastric distensibility or patients with gastric outlet obstruction due to an advanced distal tumor. Occult gastrointestinal bleeding is not uncommon and can be accompanied by iron deficiency anemia [26].
Diagnosis
Tissue diagnosis and anatomic localization of the primary tumor are best obtained by upper gastrointestinal endoscopy. Although more invasive and more costly, upper endoscopy is also more sensitive and specific for diagnosing a variety of gastric, esophageal, and duodenal lesions than alternative diagnostic strategies (such as barium studies). The early use of upper endoscopy in patients presenting with gastrointestinal complaints may be associated with a higher rate of detection of early gastric cancers.
The ability to perform biopsy during endoscopy adds to the clinical utility of this modality. As up to 5 % of malignant ulcers appear benign grossly, it is imperative that all such lesions be evaluated by biopsy and histologic assessment [27]. A barium exam may be more useful is in patients with linitis plastica. The decreased distensibility of the stiff, “leather-flask”-appearing stomach is more apparent on a radiographic study, while the endoscopic appearance may be relatively normal.
Staging
The most widely used staging system in gastric cancer was developed jointly by the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC). This classification system is most often used in the Western hemisphere and now commonly in Asian countries as well. The seventh edition was introduced in 2010 and the major differences compared to the sixth edition involved the depth of tumor invasion. Table 1 summarizes the TNM staging system for gastric cancer. Table 2 shows the 5-year survival rate according to stage.
Table 1
TNM classification of gastric cancer based on 7th Edition of the AJCC Cancer Staging Manual
T stage | N stage | M stage | Stage |
|---|---|---|---|
T0: No evidence of tumor | N0: No regional lymph node metastasis | M0: No distant metastasis | |
Tis: Carcinoma in situ | N1: 1–2 lymph nodes | M1: Distant metastasis | 0: TisNO |
Positive cytology is M1 disease | |||
T1a: Lamina propria | N2: 3–6 lymph nodes | IA: T1N0 | |
T1b: Submucosa | IB: T2N0 | ||
T2: Muscularis propria | N3a: 7–15 lymph nodes | IIA: T3N0, T2N1, T1N2 | |
N3b: >15 lymph nodes | IIB: T4aN0, T3N1, T2N2, T1N3 | ||
T3: Subserosa | IIIA: T4aN1, T3N2, T2N3 | ||
IIIB: T4bN0, T4bN1, T4aN2, T3N3 | |||
IIIC: T4bN2, T4bN3, T4aN3 | |||
T4a: Perforates serosa | IV: Any T, any N, M1 | ||
T4b: Invades adjacent organ |
Table 2
Five-year survival according to stage
Stage | 5-year survival rate (%) |
|---|---|
IA | 70.8 |
IB | 57.4 |
IIA | 45.5 |
IIB | 32.8 |
IIIA | 19.8 |
IIIB | 14 |
IIIC | 9.2 |
IV | 4 |
Clinical staging is of high importance as it dictates the management of gastric carcinoma: Stage I–III tumors are potentially curable while stage IV disease is referred for palliative therapy based on the symptoms and functional status of the patient. All stages are best managed by a multidisciplinary team.
CT scan of the chest abdomen and pelvis is performed early in the preoperative evaluation after diagnosis of gastric cancer is made. It is best suited to stage the cancer, assessing for degree of local involvement as well as for metastatic disease (hepatic or adnexal metastases, ascites). Peritoneal metastases smaller than 5 mm are frequently missed by CT scan, even when using modern CT technology [28]. Burke et al. compared staging laparoscopy to CT in 103 patients with gastric adenocarcinoma and no evidence of metastatic disease on CT scan. In 31 % of the patients, laparoscopy identified biopsy-proven metastatic disease. The sensitivity and specificity of staging laparoscopy was 94 % and 100 %, respectively [29]. Karanicolas and colleagues reviewed the SEER database to analyze frequency of staging laparoscopy in the general population in the period between 1998 and 2005. Of 6388 patients, only 506 (8 %) underwent staging laparoscopy. Use of staging laparoscopy increased over time (5.5 % in 1998 to 11.1 % in 2005, P < 0.01), and patients tended to be young and white, living in the Northeast, and with proximal cancers, and they had fewer comorbidities than those who did not undergo staging laparoscopy. Although increasing in use, staging laparoscopy seems to remain underutilized in its potential benefit of avoiding unnecessary laparotomy [30].
Endoscopic ultrasonography (EUS) is considered the most reliable nonsurgical method available for evaluating the depth of invasion of gastric cancers [31]. The accuracy of EUS for differentiation of individual tumor stages (T1–T4) ranges from 77 to 93 %, with the experience of the operator markedly influencing these rates [32]. EUS is a more accurate predictor of the T stage compared to CT [33], although the accuracy for nodal staging is only slightly greater [34]. The advantage of EUS in the assessment of the nodal status is the ability to perform fine needle aspiration of suspicious nodes and areas, which adds to the accuracy of nodal staging.
The role of positron emission tomography (PET) is still in evolution. PET is more sensitive than CT scan in the detection of distant metastases [36]. An important caveat is that the sensitivity of PET scanning for peritoneal carcinomatosis is only 50 % [37]. Therefore PET cannot adequately replace staging laparoscopy for detection of peritoneal metastases. NCCN guidelines for preoperative evaluation of gastric cancer suggest integrated PET/CT. However, gastric cancer is not a reimbursable diagnosis for PET scanning in the US Medicare program.
Extent of Gastric Resection
Distal Disease
One of the biggest controversies in surgical treatment of gastric cancer is the extent of the resection for distal lesions , i.e., whether a subtotal gastrectomy (SG) would provide similar oncologic outcomes when compared to total gastrectomy (TG). Gouzi et al. were the first to address this issue. In their prospective randomized study 169 patients underwent either SG or TG. The perioperative morbidity (34 % vs. 33 %) and mortality (3.2 % vs. 1.6 %) were similar. The overall 5-year survival was 48 %, and the extent of resection was NOT associated with different survival. Factors that determined survival were serosal invasion and lymph node involvement [38]. Even underpowered, the study showed that SG was a viable treatment option for distal lesions. A larger study by Bozzetti and colleagues included 624 patients compared patients undergoing SG (320 patients) to TG (304 patients). In this study perioperative mortality was the same between SG and TG (1.3 % vs. 2.3 %), although morbidity was greater in the TG group (15.5 % vs. 10.3 % P = 0.05). Mean length of stay was improved in the SG group (13.8 days vs. 15.4 days P = 0.001) [39] and the 5-year survival as published subsequently was comparable between the two groups (65.3 % vs. 62.4 %) [40]. One of the primary arguments in favor of SG over TG is long term quality of life. Davies and colleagues evaluated 47 consecutive patients who underwent potential R0 resection for gastric cancer. TG was performed for lesions of the proximal and middle thirds of the stomach and SG was performed for those of the distal third. D2 dissection was performed, and the spleen and pancreas were preserved when possible. No patient received adjuvant chemotherapy. Quality of life was assessed preoperatively and at 1, 3, 6, and 12 months postoperatively using five validated questionnaires. The Rotterdam symptom checklist and the Troidl index achieved a statistically significant difference between the SG and TG groups through 12 months postoperatively, with improved quality of life in the SG group as compared to the TG group [41]. Based on approximately equivalent long-term survival rates, potentially higher operative morbidity of TG, and improved quality of life for patients undergoing SG, SG is favored for distal gastric cancer, provided that adequate proximal margins of 5–6 cm are obtained.
Proximal Disease
Another controversy in gastric cancer is the oncologic and functional adequacy of proximal gastrectomy (PG) vs. total gastrectomy (TG) for malignancies of the proximal third of the stomach. Kim and colleagues retrospectively reviewed patients who underwent either PG or TG for proximal gastric cancer. PG was performed only when the cancer was limited to the proximal one-third. Between 1992 and 2000, 43 patients underwent PG and 104 underwent TG. The groups were fairly well matched for tumor characteristics, including size and differentiation, although all T4 lesions were resected via TG. The majority of the PG group underwent D1 dissection, whereas the majority of the TG group underwent at least D2 dissection. The PG group experienced higher perioperative morbidity (48.8 % vs. 14.4 %), most commonly anastomotic strictures, and higher rate of recurrence (39.5 % vs. 4.8 %). Overall 5-year survival was similar (48.6 % in TG vs. 46.0 % in PG). This survival equivalence held for stage I or stage II disease; however for stage III disease, 5-year survival after TG was significantly improved (38.4 % vs. 17.1 %). Therefore, the authors concluded that PG is best applied in early gastric cancer with achievable margins and limited nodal involvement [42].
An et al. investigated the outcomes of PG compared to TG in patients with proximal early gastric cancer. From 2000 to 2005, 423 patients underwent PG (89 patients) or TG (334 patients) for stage I or stage II proximal gastric adenocarcinoma. The TG group had larger tumors (4.0 cm vs. 2.5 cm) and more mean lymph nodes harvested (39.1 vs. 22.4). PG was associated with higher morbidity (61.8 % vs. 12.6 %), most often anastomotic stenosis and esophageal reflux, and these were successfully treated with balloon dilatation. 5-year survival was similar between the two groups (99.2 % in PG vs. 98.5 % in TG), as were long-term body weight and nutritional markers. Due to the higher perioperative morbidity the authors did not recommend proximal gastrectomy even for early gastric malignancies.
Laparoscopic Gastric Resection
After the initial introduction of laparoscopic gastrectomy for gastric cancer by Kitano et al. in 1993, the procedure has evolved significantly and is now considered one of the standard minimally invasive procedures for the treatment of early gastric cancer. There is a growing volume of literature comparing laparoscopic to open gastrectomy for early gastric cancer in the distal stomach. The first randomized studies published by Kitano, Huscher, Hayashi, and Lee were underpowered, with the number of patients recruited less than 50 [44–47], but with the same results and the same theme. The laparoscopic distal gastrectomy (LADG) group had less operative blood loss, shorter length of stay, quicker return to diet compared to the open distal gastrectomy group (ODG), and the perioperative morbidity and mortality as well as long-term survival was comparable in both laparoscopic and open gastrectomies. The largest and most notable randomized controlled study of laparoscopic vs. open distal gastrectomy for early gastric cancer is the Korean multicenter trial named KLASS (Korean Laparoendoscopic Gastrointestinal Surgery Study ). Included were patients with clinical stage I gastric adenocarcinoma. The primary endpoint was overall survival, and the secondary endpoints were disease-free survival, morbidity, mortality, quality of life, inflammatory and immune responses, and cost-effectiveness. A distal gastrectomy with D1 + β or D2 LN dissection was performed in both groups. Reconstruction was performed by Billroth I or Billroth II or Roux-en-Y fashion, depending on surgeon preference. To assure high surgical quality, surgery was performed by 15 surgeons, who had performed at least 50 cases each of LADG and ODG, at 12 high-volume institutions, which had performed more than 80 cases of distal gastrectomy per year. From February 2006 to August 2010, 1415 patients (704 LADG and 711 ODG) were enrolled, and the final results are expected to be reported in September 2015 [48]. The interim analysis of this KLASS-01 trial was published in 2010. A total of 342 patients were randomized (179 LADG and 161 ODG). There were no significant differences between the two groups concerning patient demographics. The postoperative complication rates of LADG and ODG groups were 10.5 % (17/179) and 14.7 % (24/163, p = 0.137). The postoperative mortality was 1.1 % (2/179) and 0 % (0/163) in the LADG and ODG groups (p = 0.497). The authors concluded that there was no significant difference in the morbidity and mortality between the two groups [49]. Another important Korean single-center RCT, conducted by Kim et al. [16], was published in 2008. This study aimed to evaluate the quality of life after LADG compared to ODG (n = 82 in each group) in patients with EGC. The LADG group showed better functional and symptom scales of EORCT QLQ-C30 and QLQ-STO22 at 3 months after surgery. Also, intraoperative blood loss, total amount of postoperative analgesics, and postoperative hospital stay were significantly less in the LADG group. The authors concluded that LADG resulted in improved quality of life outcomes after surgery in EGC patients compared to ODG. To evaluate the long term results of LADG Strong et al. from Memorial Sloan-Kettering Cancer Center reported a retrospective case–control study comparing 30 LADG with 30 ODG. Controls were matched for stage, age, and gender from 2005 to 2008. The mean number of resected LNs was 18 (range: 7–36) in the LADG group and 21 (range: 7–44) in the ODG group (p = 0.03). There were four recurrences (13.3 %) in the LADG group during 11 months of follow-up and five recurrences (16.6 %) in the ODG group during 13.8 months of follow-up (p = 0.71).
Laparoscopic Gastrectomy for Advanced Gastric Cancer
As the sophistication of available technology has improved and laparoscopic experience has evolved, laparoscopic resections with extensive lymphadenectomy for advanced gastric cancer (AGC) have emerged. A recent meta-analysis, including seven case–control studies with 1271 AGC patients (626 LADG and 645ODG), showed that LADG patients had longer operative time but less estimated blood loss, less analgesic requirement, and a shorter hospital stay compared with patients undergoing ODG. There were no significant differences between the two groups in number of LN harvested, postoperative mortality, overall complications, and 3-year overall survival rate. Therefore, the authors concluded that the oncologic outcomes of LADG for AGC patients were comparable with an open approach [50]. Based on this data the KLASS two trial was launched in October 2011 to compare LADG to ODG for advanced gastric cancer. The estimated sample size is 1050 and the primary endpoint is 3-year disease-free survival rate. As the surgical quality may emerge as an important issue in this clinical trial, surgeons are required to undergo a quality assessment before joining the trial. On February 2013, 18 surgeons at 11 institutes had been approved, and 316 patients out of 1050 (30.1 %) were enrolled for the last year. The results of this trial are eagerly anticipated.
Extent of Lymph Node Dissection
One of the most controversial areas in the surgical management of gastric cancer is the optimal extent of lymph node dissection. Japanese surgeons routinely perform extended lymphadenectomy, a practice that some suggest at least partially accounts for the better survival rates in Asian as compared to Western series. The term “extended lymphadenectomy” variably refers to either a D2 or a D3 lymph node dissection. Table 3 shows the different gastric lymph node stations, divided according to the Japanese classification [51]. Stations 1–6 are perigastric, and the remaining ten are located adjacent to major vessels, behind the pancreas, and along the aorta.
Table 3




Gastric lymph node stations, according to Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011 Jun;14(2):101–12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree