BTK Inhibitors
Blake Elizabeth Brooks
Elisa M. Schunkert
Sherrie J. Divito
Bruton tyrosine kinase (BTK) is a cytoplasmic nonreceptor tyrosine kinase that plays an integral role in signal transduction of a variety of cell surface receptors in both normal and malignant cells. BTK is primarily expressed in hematopoietic cells, with high levels of expression in B cells, myeloid cells, and platelets, but low or absent expression in T cells and plasma cells.1 BTK inhibitors (BTKi) hinder B cell survival, proliferation, and differentiation by impeding BTK activation after B cell receptor-antigen engagement1 and modulate B cell motility and homing by interfering with chemokine receptor- and integrin signaling.1 Second-generation inhibitors exhibit higher target selectivity than first-generation drugs, thereby theoretically mitigating inhibition of off-target kinases.1 However, reported dermatologic toxicities in phase II/III trials of second-generation BTKi were largely comparable to those of first-generation BTKi,2 though in practice appear less severe. Also, toxicities from prior first-generation BTKi therapy can recur in patients who initiate treatment with second generation BTKi.2 FDA-approved inhibitors include ibrutinib (first generation), acalabrutinib (second generation), and zanubrutinib (second generation), though there are more currently in phase II/III clinical trials.
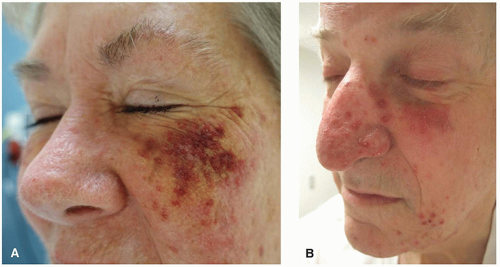
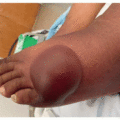
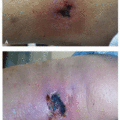
Dermatologic manifestations are one of the most commonly cited adverse effects of BTKi and are wide ranging in their presentation. Hemorrhage into the skin is one of the most common dermatologic toxicities reported, occurring in approximately a third of patients.2,3 Lesion morphology varies, and can include petechiae, ecchymoses, purpura, hematomas, hemorrhagic crusting or vesicles, and target-like lesions.2,3 Importantly, patients may bleed into any inflammatory process in the skin, making the eruption appear purpuric (Figure 34.1).
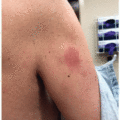
BTKi most commonly induce a papular eruption with a predilection for the lower extremities (Figure 34.2). Though purpuric and palpable, it is generally not associated with vasculitis on biopsy and is topical and systemic steroid responsive.4
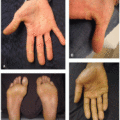
Many BTKi cutaneous toxicities resemble those seen with EGFR inhibitors and are hypothesized to be associated with off-target EGFR inhibition. These include photosensitivity, an acneiform rosacea-like eruption (Figure 34.1B), xerosis, and hair and nail changes. Xerosis can be associated with eczematous changes (Figure 34.3A and B).3 Pityriasis rosea-like eruptions have also been reported (Figure 34.3C).2,5 Immune-mediated drug reactions such as morbilliform drug eruption can occur; however, severe cutaneous adverse reactions (SCARs) including drug reaction with eosinophilia and systemic symptom, toxic epidermal necrolysis, and acute generalized exanthematous pustulosis have not been reported.2
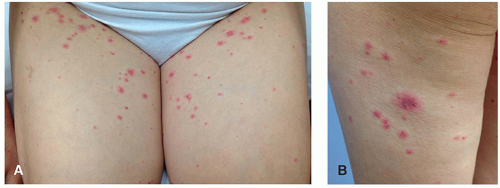 Figure 34.2. Papular eruption associated with BTKi that may be pink or purpuric and resemble leukocytoclastic vasculitis on the upper thighs (A) and posterior leg (B) of a patient on ibrutinib. On biopsy, LCV is not typically present and the “palpable purpura” presentation is felt to be due to platelet dysfunction and a purple appearance of what would otherwise be pink. Patients respond to topical or systemic steroids. (Photos courtesy of Bernice Kwong, MD.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|