Hodgkin lymphoma (HL) is a relatively rare but highly curable human cancer. Because very few patients relapse and will require subsequent therapy, new drug development for HL has not been seen as a high priority by the pharmaceutical industry. Brentuximab vedotin, an antibody-drug conjugate that targets CD30 receptors, became the first drug to be approved by regulatory agencies for the treatment of HL in more than 30 years. This review summarizes the current and future directions of incorporating brentuximab vedotin in the management of patients with HL.
Key points
- •
Brentuximab vedotin, an antibody-drug conjugate that targets CD30, is one of the most active single agents for the treatment of patients with relapsed classic Hodgkin lymphoma.
- •
Brentuximab vedotin should not be combined with bleomycin, as the combination can cause excessive pulmonary toxicity. When combined with front-line ABVD, bleomycin is eliminated from the regimen.
- •
The most common brentuximab vedotin toxicity is cumulative but reversible neuropathy.
- •
Brentuximab vedotin can be safely administered before and after allogeneic stem cell transplant.
Introduction
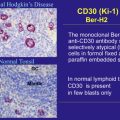
CD30 is considered an ideal target for monoclonal antibody therapy for Hodgkin lymphoma (HL), because its expression is highly restricted to the malignant Hodgkin and Reed-Sternberg (HRS) cells. CD30 is a transmembrane receptor that belongs to the tumor necrosis factor receptor superfamily. In addition to its membrane-bound form, CD30 can also be shed in a soluble form. Over the past 2 decades, several investigators have evaluated the safety and efficacy of a wide range of monoclonal antibodies targeting CD30 in patients with relapsed HL. Results from clinical trials using a variety of naked monoclonal antibodies targeting CD30 have demonstrated an excellent safety profile, but with limited antitumor activity ( Table 1 ). These disappointing clinical results could be attributed to poor antigen-binding properties of these antibodies, ineffective activation of effector cells, and/or neutralization by high levels of soluble serum CD30.
| Drug | Phase of Study | No. of Evaluable Patients | PR | CR | PR + CR |
|---|---|---|---|---|---|
| MDX-060 | II | 63 | 2 | 2 | 4 (6%) |
| SGN-30 | II | 38 | 0 | 0 | 0 |
| Xmab2513 | I | 13 | 1 | 0 | 1 (7%) |
| SGN-35 (every 3 wk) | I | 42 | 7 | 10 | 17 (40%) |
| SGN-35 (weekly) | I | 35 | 10 | 6 | 16 (46%) |
| Brentuximab vedotin | II | 102 | 41 | 35 | 76 (75%) |
CD30 is internalized, making it a suitable target for antibody-drug conjugate (ADC) treatment strategies. Earlier, custom-made ADCs demonstrated clinical efficacy but also resulted in significant toxicity. More recently, the naked antibody SGN30 was linked to the antitubulin monomethyl auristatin E (MMAE), to generate the ADC brentuximab vedotin (formerly known as SGN35).
Introduction
CD30 is considered an ideal target for monoclonal antibody therapy for Hodgkin lymphoma (HL), because its expression is highly restricted to the malignant Hodgkin and Reed-Sternberg (HRS) cells. CD30 is a transmembrane receptor that belongs to the tumor necrosis factor receptor superfamily. In addition to its membrane-bound form, CD30 can also be shed in a soluble form. Over the past 2 decades, several investigators have evaluated the safety and efficacy of a wide range of monoclonal antibodies targeting CD30 in patients with relapsed HL. Results from clinical trials using a variety of naked monoclonal antibodies targeting CD30 have demonstrated an excellent safety profile, but with limited antitumor activity ( Table 1 ). These disappointing clinical results could be attributed to poor antigen-binding properties of these antibodies, ineffective activation of effector cells, and/or neutralization by high levels of soluble serum CD30.
| Drug | Phase of Study | No. of Evaluable Patients | PR | CR | PR + CR |
|---|---|---|---|---|---|
| MDX-060 | II | 63 | 2 | 2 | 4 (6%) |
| SGN-30 | II | 38 | 0 | 0 | 0 |
| Xmab2513 | I | 13 | 1 | 0 | 1 (7%) |
| SGN-35 (every 3 wk) | I | 42 | 7 | 10 | 17 (40%) |
| SGN-35 (weekly) | I | 35 | 10 | 6 | 16 (46%) |
| Brentuximab vedotin | II | 102 | 41 | 35 | 76 (75%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree