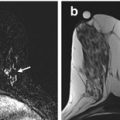
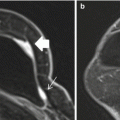
Fig. 13.1
Fifty-five-year-old asymptomatic woman undergoing screening breast MRI for elevated lifetime risk of breast cancer due to family history. (a) Axial T1-weighted post-contrast images demonstrated a 5 mm oval mass (white arrow) with circumscribed margins and heterogeneous internal enhancement in the right breast at 5 o’clock middle depth with initial rapid and delayed washout kinetics (BI-RADS® 4A). Targeted ultrasound showed no correlate. (b) MRI-guided biopsy was performed of the right breast mass (white arrow) using a medial approach with ten specimens obtained from a 9 gauge vacuum-assisted device. (c) Axial contrast-enhanced post-biopsy sequences demonstrated hematoma at the expected site of biopsy. Histopathology results were benign (breast tissue with cysts, fibrosis, apocrine metaplasia, and usual ductal hyperplasia) and concordant. (d) Six-month follow-up MRI was recommended which demonstrated susceptibility artifact from the biopsy clip and no residual enhancing mass (BI-RADS® 2)
The purpose of determining concordance is to minimize the potential for false-negative biopsies resulting from inadequate sampling or inaccurate targeting and to avoid a delayed diagnosis of cancer. The frequency of inadequate tissue sampling of MRI lesions has been reported as 6–14 % [13–15]. Use of vacuum-assisted devices, typically with 9 gauge needles, are encouraged which yield generous amounts of tissue for thorough histopathologic examination [7]. As for other breast imaging modalities, when a malignancy is detected within one year at the site of a benign MRI-guided biopsy it is considered a false-negative [5]. False-negative rates for MRI-guided breast biopsies range from 0.9 to 11.7 % [10, 13–17]. Accurate determination of a program’s false-negative biopsy rate is inherently challenging due to the potential lack of patient follow-up at the same institution. Audit data linkage with state or regional cancer registries can be helpful to improve the accuracy of false-negative biopsy rate.
Information used in determination of radiologic-pathologic concordance starts at the time of the diagnostic examination. A BI-RADS® assessment of 5 (highly suggestive of malignancy) indicates that a benign biopsy result should, in most instances, be deemed discordant. Furthermore, subcategorization of BI-RADS® 4 assessments into 4A, 4B, and 4C (low, moderate, and high suspicion for malignancy, respectively) is also informative. In contrast to mammography and ultrasound, subcategories for BI-RADS® 4 assessments are not included for MRI in the most current edition of the BI-RADS® Atlas [5]. However, subcategorization of BI-RADS® 4 assessments can be particularly useful for breast MRI radiologic-pathologic correlation because the level of concern for malignancy is more stratified. For example, a lesion with a 4C assessment that yields benign biopsy results should be reviewed with particular scrutiny. Particular benign pathologies that are well-known to present as suspicious imaging findings, such as fat necrosis, could be considered concordant in these instances.
Assessing the adequacy of tissue sampling at the time of biopsy also contributes to concordance determination. Immediate post-biopsy images are obtained and reviewed during the procedure to allow for adjustment and additional sampling if needed. Some practices perform a second injection of contrast to revisualize the lesion [11]. However, the presence of blood and air in the biopsy cavity frequently limits the utility of this approach.
Adequate communication with the interpreting pathologist is another key factor in optimizing radiologic-pathologic concordance. Inclusion of key clinical information, indication for biopsy, imaging features of the biopsied lesion, potential differential diagnoses based on imaging, and the BI-RADS® assessment on the pathology requisition form provides a quick and focused method for conveying this important information. For complicated cases or those with unusual or unexpected histopathologic results, the pathologist may contact the radiologist who performed the procedure with specific questions before issuing their final report. Being available and engaged in these conversations further improves radiologic-pathologic concordance and strengthens the multi-disciplinary approach to patient care.
Once biopsy results are issued by pathology, the methods used for assessing radiologic-pathologic concordance vary by institution and practice type. One approach involves a dedicated multidisciplinary clinical conference. The radiologist presents the clinical history and imaging studies performed before, during, and after the biopsy to demonstrate initial findings and level of suspicion for malignancy, adequate targeting and sampling, and appropriate marker clip placement. This is followed by presentation of the histopathologic results by the pathologist. Group consensus is reached regarding concordance, and management recommendations are determined. This method can foster interdepartmental professional relationships and can be achieved in this modern electronic era through remote Picture archiving and communication systems (PACS) and scanned histology slides through programs available on the internet and/or video-conferencing. An approach such as this may be more amenable to implementation at teaching institutions. For settings in which it might not be practical for a physical radiology-pathology correlation conference such as high-volume clinical services, the radiologist may perform dedicated review of imaging findings independently or together with other radiologists in the group using the written pathology report.
Determining radiologic-pathologic concordance relies upon knowledge of the acceptable histopathology for particular imaging findings. For breast MRI, most of the research has focused on the imaging features that are predictive of malignancy. For example, foci have been shown to have lower probabilities of malignancy compared to masses or non-mass enhancement [18]. For masses on MRI, margins have been found to be an important imaging predictor [19, 20]. However, there are relatively few data regarding the MRI features that are associated with particular benign histopathology outcomes. Biopsies of breast MRI findings have been shown to result in a spectrum of benign, concordant histopathology results. These include nonspecific findings such as fibrocystic change, sclerosing adenosis, fibrosis, pseudoangiomatous stromal hyperplasia, and normal breast parenchyma [20, 21]. More specific benign and concordant results include fibroadenoma, papilloma, and lymph node. In general, nonspecific results have been more frequently associated with non-mass enhancement [20, 21], but further studies are warranted to clarify acceptable MRI lesion and histopathology outcomes.
Once radiologic-pathologic correlation has been performed and concordance has been determined, management recommendations are made and communicated to the referring physician and the patient. Patients with malignant results are referred to a breast surgeon and/or medical oncologist for treatment. Management of patients with benign results that are discordant and those with benign results that are concordant are discussed in the subsequent sections of this chapter. Importantly, an addendum is made to the original biopsy report with the histopathologic results, radiologic-pathologic concordance, and management recommendations.
Practice guidelines regarding MRI-guided breast biopsy procedures have been published by the American College of Radiology (ACR) and as a report from a European interdisciplinary consensus meeting [6, 7]. The ACR states that the physician who performed the procedure “is responsible for obtaining results of the histopathologic sampling to determine if the lesion has been adequately biopsied and is concordant or discordant with the imaging findings” [7]. The European interdisciplinary consensus report recommends “all available clinical and imaging information and VAB results be compared and discussed in an interdisciplinary conference to achieve a consensus recommendation in each case” [6]. These reports reinforce the importance of assessing concordance.
For several reasons, radiologic-pathologic concordance is more challenging for MRI-guided biopsies compared to stereotactic- and ultrasound-guided biopsies. First, there is no specimen radiograph to confirm adequate sampling due to the lack of tissue enhancement ex vivo. Second, there is no “real-time” visualization of the needle at the time of tissue sampling since the biopsy is performed when the patient is outside of the magnet. Determining whether the targeted finding has been appropriately sampled on post-biopsy MRI sequences has limitations as lesions with wash-out contrast kinetics become less conspicuous over time while enhancement of normal breast parenchyma increases. Also, lesions can be obscured by hematoma and air on post-biopsy sequences. These factors together with the higher pre-test probability of malignancy in women undergoing breast MRI support adopting a careful approach to radiologic-pathologic concordance to avoid a delayed cancer diagnosis.
13.3 Discordant MRI-Guided Breast Biopsy Results
A discordant biopsy result is one in which the histopathology does not sufficiently explain the imaging findings [12]. The discordance rates for MRI-guided breast biopsies using vacuum-assisted devices range from 0 to 9 % [9, 10, 22–26]. The rates of discordant biopsies are higher for MRI-guided biopsies compared with stereotactic- or ultrasound-guided biopsies (approximately 3 %) [12, 24]. Interestingly, discordance has not been shown to occur more often with BI-RADS® category 5 compared with category 4 lesions or to occur more often for radiologists with less experience with MRI-guided biopsies, factors that are known to affect discordance rates for stereotactic- and ultrasound-guided biopsies [24].
Further tissue sampling is warranted in cases of discordant MRI-guided biopsy results (see Fig. 13.2) [6, 7]. Options include repeat MRI-guided biopsy or surgical excision. The method used for preoperative wire localization prior to surgical excision includes mammographic-guidance if the marker clip placement is deemed appropriate. If there is significant clip displacement and mammographic landmarks are lacking, MRI-guided wire localization can be performed. The malignancy rate for discordant lesions that subsequently undergo surgical excision is 30–50 % [22, 24]. Thus, appropriate recognition and management of discordant lesions is clinically significant.
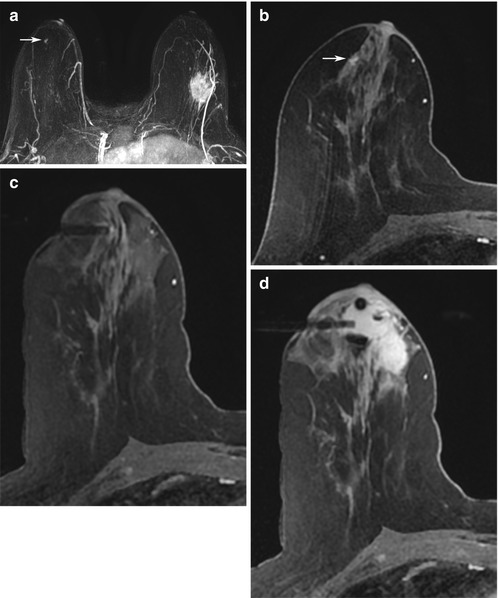
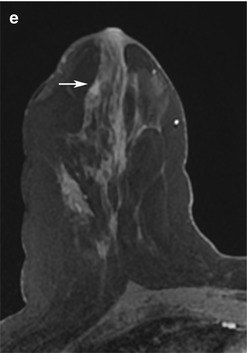


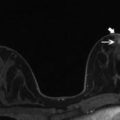
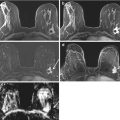
Fig. 13.2
Forty-nine-year-old woman with newly diagnosed left breast cancer undergoing preoperative breast MRI for extent of disease evaluation. (a) Maximum intensity projection images demonstrate the biopsy-proven malignant mass in the left breast and a 6 mm irregular mass (white arrow) with irregular margins and homogeneous internal enhancement in the right breast at 9 o’clock anterior depth with initial rapid and delayed plateau kinetics (BI-RADS® 4B). Axial T1-weighted post-contrast images of the right breast mass (white arrow) are shown in (b). (c) MRI-guided biopsy was performed of the right breast mass using a lateral approach with 8 specimens obtained from a 9 gauge vacuum-assisted device. Preferential sampling was performed in the superior and lateral directions to account for patient motion noted after targeting. (d) Post-biopsy hematoma was located in the expected site of biopsy. Histopathology results were benign breast tissue. The anterior location of the lesion and relative lack of sufficient compression to prevent motion were inherent technical challenges encountered since the patient was undergoing bilateral MRI-guided breast biopsies for an additional lesion in the left breast located at middle to posterior depth. (e) Review of post-biopsy images demonstrated a persistent enhancing mass (white arrow) indicating insufficient tissue sampling. The benign biopsy result was deemed discordant and repeat MRI-guided biopsy of the right breast was performed with more anterior compression. Histopathology results were ductal carcinoma in situ, low grade, ER+PR+
For discordant lesions undergoing repeat MRI-guided biopsy, radiologic-pathologic concordance should again be determined. Similarly, review of final histopathologic results for cases recommended for surgical excision are informative and recommended [6]. Important factors to note include the presence or absence of prior biopsy site changes in the excised specimen and whether any residual lesion exists in the specimen as well as final histopathologic size since small lesions may be completely removed during the biopsy procedure.
13.4 Management Recommendations for Patients with Benign Concordant Biopsy Results
Due to the challenges involved in confirming adequate sampling at the time of the MRI-guided biopsy procedure, a follow-up MRI examination is recommended for patients with benign concordant biopsy results to identify any delayed false-negative cases. The overall cancer yield at follow up-MRI has been reported as 0.9–2.3 % [13, 16, 17, 20]. The recommendation for follow-up MRI also includes when biopsies of suspicious MRI findings are performed using ultrasound guidance of presumed correlates identified on MRI-targeted ultrasound. The rationale for this recommendation is based on the results of Meissnitzer et al. which demonstrated that the presumed correlate on ultrasound did not correspond to the MRI finding of concern in 12.5 % of cases (10/80) with 5 cancers diagnosed in 9 lesions that underwent subsequent MRI-guided biopsy [27].
Ideally, the follow-up examination should be performed at the same institution using the same imaging acquisition protocol to best evaluate for potential interval change. Two studies have described an increase in the largest lesion dimension by 10 % as evidence of an interval size change, but there is no standardized definition for what constitutes clinically significant change [13, 16]. Lesions demonstrating concerning enlargement or development of more suspicious imaging features should undergo repeat biopsy or surgical excision (see Fig. 13.3) [16]. If the biopsied lesion decreases in size or resolves completely on the follow-up MRI, adequate sampling is confirmed and no further surveillance is required [15, 16]. This approach is supported by data of Dratwa et al. that showed no interval change at a 12 month follow-up MRI for 117 benign concordant lesions that had decreased or resolved at the initial 6 month examination [20].
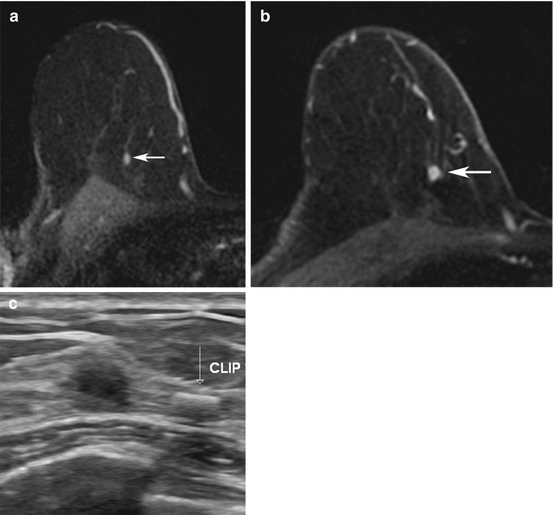

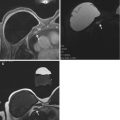
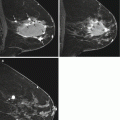
Fig. 13.3




Seventy-four-year-old woman with a personal history of prior treated right breast cancer and BRCA1 gene mutation undergoing asymptomatic screening breast MRI. (a) Axial T1-weighted post-contrast images demonstrated a new 3 mm focus of enhancement (white arrow) in the right breast at 1 o’clock posterior depth with initial rapid and delayed plateau kinetics (BI-RADS® 4A). MRI-guided biopsy was performed and ten specimens were obtained using a 9 gauge vacuum-assisted device. Histopathology results were benign and concordant. Six-month follow-up MRI was recommended. (b) Axial T1-weighted post-contrast images demonstrated a 6 mm round mass with a circumscribed margin and homogeneous enhancement (white arrow) in the right breast at 1 o’clock posterior depth with initial rapid and delayed plateau kinetics (BI-RADS® 4B). Susceptibility artifact from the previous placed MRI-guided biopsy clip was present along the posterior aspect of the mass. (c) Targeted ultrasound demonstrated an irregular hypoechoic mass with indistinct margins which correlated with the mass seen on MRI. A biopsy clip was noted adjacent to the mass. Histopathology results from ultrasound-guided biopsy were invasive ductal carcinoma, grade 2, ER-PR-HER2-
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree