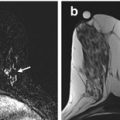
Fig. 6.1
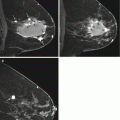
(a) Axial STIR with water suppression (silicone sensitive) MRI image showing bilateral intact silicone implants. Signal from the saline component of the phantom (white arrow) is suppressed. High intensity signal from the silicone component of the phantom (black star) matches the high signal of the silicone implants. (b) Axial STIR with silicone suppression MRI image showing bilateral intact silicone implants. There is high intensity signal from the saline component of the phantom (white arrow). Suppressed signal from the silicone component of the phantom (white star) matches the suppressed signal of the silicone implants
Less commonly encountered is the standard double-lumen implant, which consists of a silicone gel filled inner lumen and a saline outer lumen. The primary purpose of this implant type is to allow size adjustability at the time of and after implant placement. A reverse double lumen implant, most commonly used after reconstructive surgery, consists of a saline filled inner lumen and silicone gel outer lumen. Size adjustments can be made by adding saline to the inner lumen while preserving the feel of a silicone gel-filled implant. A gel-gel double lumen implant consists of silicone gel within the inner and outer lumens.
Additional more rarely encountered implants on the market include reverse-adjustable, triple-lumen, double lumen Cavon “cast gel”, custom, soft pectus, non-adjustable sponge, adjustable sponge and others [2].
Breast implants can be placed behind the glandular tissue but anterior to the pectoralis major muscle (termed subglandular, retroglandular or retromammary position). This position maximizes the augmentation effect of the implant, but obscures more breast tissue on mammogram, limiting evaluation. Alternatively, implants may be placed posterior to the pectoralis muscle (termed subpectoral or retropectoral position); this is the case for all implants placed after total mastectomy [6]. After placement, a thin fibrous capsule of scar tissue normally forms around the implant. On occasion, pronounced fibrous capsule formation can occur with silicone implants, which causes discomfort and alters the shape of the breast. This is known as capsular contracture and can be difficult to diagnose by imaging. Although surgically more challenging to place, advantages of subpectoral implants include lower rate of capsular contracture and easier imaging of the surrounding breast tissue [1].
6.3 Imaging of Implants
Breast augmentation is the most common cosmetic surgical procedure performed in the United States, and has been since the FDA re-approved the use of silicone implants in 2006. In 2014 there were 286,254 cases of breast augmentation with implants reported, a 35 % increase since 2000 [7]. With the widespread prevalence and ever increasing number of women with implants, there is an ongoing need to evaluate breast implant integrity as well as to identify breast cancer in women with implants.
6.4 Mammography
The primary indication for performing mammography in women with implants is to detect breast cancer. Conventional mammography is of little value in the assessment of implant integrity, with sensitivity ranging from 25 to 68 % [8] (Fig. 6.2). The sensitivity of screening mammography for detecting malignancy is also decreased in the presence of implants and has been reported at 45 % versus 67 % in patients without implants [9]. Mammography does however remain useful for the evaluation of the surrounding breast tissue and for the detection of extracapsular silicone rupture. Additionally, mammography can identify periprosthetic calcifications, which are occasionally seen with capsular contracture as well as focal bulges or contour deformity of the implant shell. Given that mammography is the main screening tool to identify breast cancer in women, annual mammography is still recommended in patients with implants.
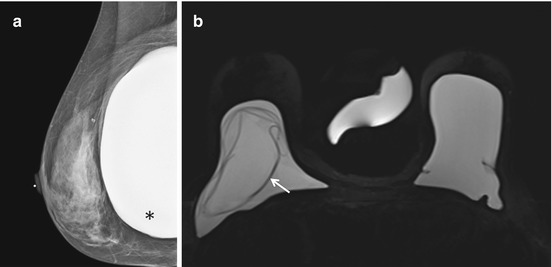

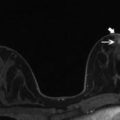
Fig. 6.2
(a) Right MLO view mammogram shows a subpectoral silicone implant, which appears intact (black asterix). (b) Axial STIR with water suppression (silicone sensitive) MRI image from the same patient demonstrates the “linguine sign” (white arrow) of intracapsular implant rupture on the right, which was not detected on mammography performed the same day
6.5 Ultrasound
There are conflicting reports on the usefulness of ultrasonography for detecting implant ruptures, which may reflect the variation in exam quality depending on the experience of the operator, type of equipment used, and technical factors [8]. Ultrasound can delineate some of the internal structure of the implant, particularly in the anterior aspect and therefore can detect both intracapsular and extracapsular rupture. Intracapsular rupture is seen as a series of horizontal echogenic straight or curvilinear lines traversing the interior of the implant, commonly known as the stepladder sign [10] (Fig. 6.3). Extracapsular silicone has the characteristic “snowstorm” appearance characterized by a highly echogenic pattern of scattered and reverberating echoes with a well-defined anterior margin and loss of detail posteriorly (Fig. 6.4). Ultrasound is also able to detect small amounts of free silicone within axillary lymph nodes, manifesting as the characteristic echogenic snowstorm appearance.
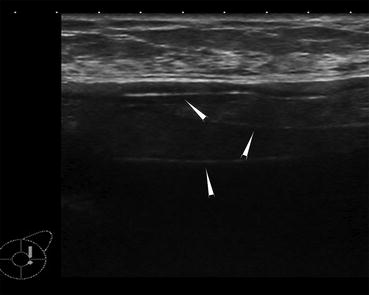
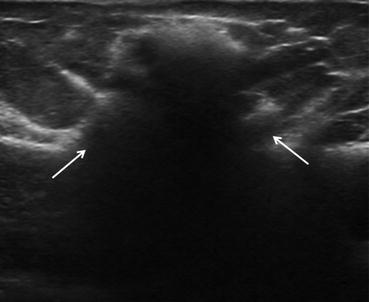

Fig. 6.3
Image from an ultrasound demonstrates the stepladder sign of intracapsular silicone implant rupture (white arrowheads). The discontinuous parallel echogenic lines represent the collapsed implant shell within the implant lumen

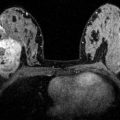
Fig. 6.4
Image from an ultrasound demonstrates the “snowstorm” pattern of extracapsular silicone implant rupture (white arrows)
Ultrasound has proven to be useful in patients who are claustrophobic or unable to undergo MRI because of unsafe implanted devices such as pacemakers. Importantly however, silicone does cause marked attenuation of the ultrasound beam; thus evaluation of the back wall of an implant and the tissue posterior to it is limited. Additionally, previous silicone injections and residual silicone granulomas from extracapsular rupture will significantly limit ultrasound evaluation [11].
6.6 Magnetic Resonance Imaging
Magnetic resonance imaging is the most accurate noninvasive method available to evaluate silicone gel-filled implant integrity. The FDA currently recommends that asymptomatic women with silicone implants undergo an MR imaging examination to check for implant rupture 3 years after placement and then every 2 years thereafter. In addition, MR imaging is recommended to evaluate for implant rupture if the patient is having new breast symptoms [12]. This is because although clinical signs of implant rupture may include contour deformity, displacement, and mass formation, the diagnosis of implant rupture based on physical exam findings is extremely insensitive, with failure to diagnose implant rupture in greater than 50 % of ruptures [13]. The overall sensitivity of MRI for detection of implant rupture is between 80 and 90 % and its specificity is between 90 and 97 % [14].
6.6.1 MRI Sequences
Currently breast implant imaging can be performed on 1.5 and 3 T MR scanners from all major manufacturers using a dedicated breast imaging coil. Studies are performed in the prone position to minimize respiratory artifact and to allow the implant to be imaged in maximum size. The MR sequences that are used in silicone implant imaging are strategically developed to separate three main components: water, silicone and fat [4].
We find imaging of a saline bag/silicone phantom to be helpful both in terms of aiding confirmation of implant material, but also as a quality control measure for signal suppression (Fig. 6.1). Implant sequences can be performed using 5 mm slices and can be performed in under 15 minutes; however, a longer high resolution sequence may be useful for thin shell and some standard double-lumen implants when early rupture detection is difficult.
We suggest that MRI to evaluate for implant rupture should include a scout localizer sequence, to help plan other sequences. The scout sequence is useful for example, in the rare situation when extracapsular silicone has spread outside the normal field of view (FOV), and can be utilized to plan extended FOV studies. A bilateral 2D axial T2-weighted turbo spin-echo sequence is used to differentiate water from silicone in cases of failure of nulling or suppression.
A bilateral 2D axial short tau inversion recovery sequence with water saturation is the main sequence used to detect intracapsular and extracapsular rupture of silicone implants. In this sequence silicone will appear hyperintense against a dark background of fat and water suppressed images. The dark implant shell folds will contrast with the bright silicone gel in cases of intracapsular rupture.
A bilateral 2D axial short tau inversion recovery sequence with silicone saturation is used to increase confidence in detecting extracapsular soft-tissue silicone. In this sequence, water and fat will appear hyperintense, and silicone will be hypointense, and thus extracapsular silicone will appear dark against a bright background. This sequence is not used to detect intracapsular implant rupture because the hypointense implant shell folds are not well seen against the background of suppressed (hypointense) silicone gel.
Additional sequences used to evaluate implant integrity at our institution include a bilateral 2D axial STIR, bilateral 2D axial T2 and a bilateral 2D axial T1-weighted volumetric interpolated breath-hold examination (VIBE) (see Table 6.1).
Table 6.1
Protocol for implant evaluation (3 T magnet)
Description | Main parameters | Uses |
|---|---|---|
Bilateral 2D axial T2-weighted unsupressed, breast coil | Turbo spin-echo, 5 mm slice thickness, TR 3000 ms, TE 79.0 ms, 384 × 288 matrix, 1 average | Allows differentiation of water from silicone in cases of nulling/suppression failure |
Bilateral 2D axial STIR, breast coil | Turbo spin-echo, 5 mm slice thickness, TR 3500 ms, TE 61.0 ms, 320 × 256 matrix, 2 averages | Used in conjunction with unsuppressed T2 to confirm fat signal, which will be hypointense on this sequence |
Bilateral 2D axial STIR, silicone saturated, breast coil | Turbo spin-echo, 5 mm slice thickness, TR 4000 ms, TE 61.0 ms, 320 × 256 matrix, 2 averages | Used to confirm the presence of extracapsular silicone, which will appear hypointense |
Bilateral 2D axial STIR, water saturated, breast coil | Turbo spin-echo, 5 mm slice thickness, TR 4000 ms, TE 61.0 ms, 320 × 256 matrix, 2 averages | Main sequence to detect intracapsular and extracapsular implant rupture |
Bilateral 2D axial T1- weighted VIBE, breast coil | Gradient echo, 0.9 mm slice thickness, TR 3.78 ms, TE 1.11 ms, 448 × 358 matrix, 1 average | High resolution sequence useful for thin shell and some standard double-lumen implants when early rupture detection is difficult. Can also be used to evaluate non-implant related breast findings |
6.7 Implant Integrity/Complications
The normal silicone implant should have a smooth, well-defined margin with homogeneous appearance of silicone on MRI. Breast silicone implants are designed to approximate the cosmetic ptosis of a normal breast and, thus are not meant to be taut. This means when an under-filled implant is placed into a confined space, it in-folds on itself as needed to conform to the space, and as such minor rippling or undulation is a normal finding on MR imaging. This commonly seen fold pattern can cause confusion as it mimics implant rupture; however normal folds should extend from the edge of the implant shell inward (Fig. 6.5). Additionally, no silicone should ever be seen outside the implant as a whole, along the inner surface of the fibrous capsule or within the folds themselves.
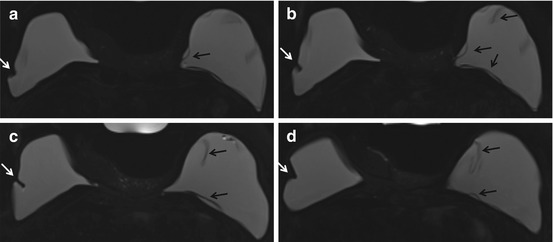

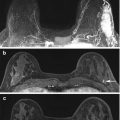
Fig. 6.5
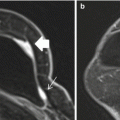
Axial STIR with water suppression (silicone sensitive) MRI image demonstrates a radial fold (white arrow) on multiple slices of the right breast implant (a–d). When scrolling through the entire sequence, the radial fold extends to the surface of the implant shell. This is in contrast to the hypointense curvilinearities signaling intracapsular rupture (“linguine sign”) seen in the left implant (black arrows) (a–d)
6.7.1 Intracapsular Rupture
When breast implants fail, the most common cause is a small defect or worn area of the implant shell. A large percentage of implant failures occur during surgical implantation; however tears can also occur in-vivo. While trauma can cause an implant to rupture, most implant ruptures have no identifiable cause. The most important factor predisposing an implant to rupture is age of the implant. Historically the prevalence of implant rupture is approximately 30 % at 5 years after implantation, 50 % at 10 years and 70 % at 17 years, with the median age of implants at rupture being around 10.8 years [15]. Additional studies, however, have shown lower rates of implant rupture with later-generation silicone implants [16–18].
When there is a defect in the implant elastomer shell, silicone gel will slowly ooze out, but will be contained in the intracapsular space by the outer fibrous capsule. Over time the escaped silicone contained by the fibrous capsule will surround the implant elastomer shell and cause it to collapse into the pool of remaining silicone.
Four categories of implant intracapsular rupture have been described, from early to advanced; these include uncollapsed, minimally collapsed, partially collapsed and fully collapsed [19]. Describing intracapsular rupture by stage using these common terms can be helpful and informative for surgeons and patients when making clinical decisions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree