Source
Number of patients
Additional cancer yield of MR (%)
MR sensitivity (%)
MR specificity (%)
Mammographic sensitivity (%)
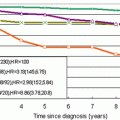
Kriege
1,909
2.4
71.1
89.8
33
Warner
236
9.3
85
93
36
MARIBS
649
2.9
77
81
40
Kuhl
192
3.0
100
75
33
Lehman
367
1.1
100
94
25
Podo
105
4.8
100
99
13
Dense Breasts
Mammography performs poorly in women with dense breasts [15]. This problem led some health care organizations to recommend against screening mammography in women under 50 [16, 17].
There is evidence in the non-MR literature that indicates impaired staging in women with dense breasts. Women with dense breasts are more likely to fail breast conservation due to occult disease. A study showed that 10-year local recurrence rate for women with dense breasts was 21 % compared to 5 % for low density (hazard ratio of 5.7). This difference was greater in women who did not receive radiotherapy with recurrences in 40 % of the dense breast group compared to none with less than 25 % density [18]. Another study from UCSF showed similar findings. Women with dense breasts (greater than 75 % density) had a hazard ratio of 4.3 for local recurrence compared to low-density women (less than 25 % density) [19]. Morrow’s group recently translated this experience to actual practice where they showed that women with radiographically dense breasts are more likely to be treated with mastectomy (61 %) compared with women with lesser density (43 %). Failure of breast conservation was due to luminal A tumors, lobular histology, multicentricity, and occult tumors [20]. Patients where the original tumor was mammographically occult are more likely to have diffuse histology and more difficult to follow [21].
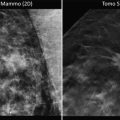
A recent study from Canada comparing mammographic and MR screening in high-risk women showed mammographic sensitivity in women with greater than 50 % breast density to only be 12 %, compared with MR sensitivity in the same group of 75 %. However, even in women with the lowest breast density (less than 10 % density) mammographic sensitivity only improved to 31 %, compared to 92 % sensitivity for MR [22]. An example of an incidentally detected cancer on MR in a woman with extensive fibrocystic changes is shown in Fig. 8.1.
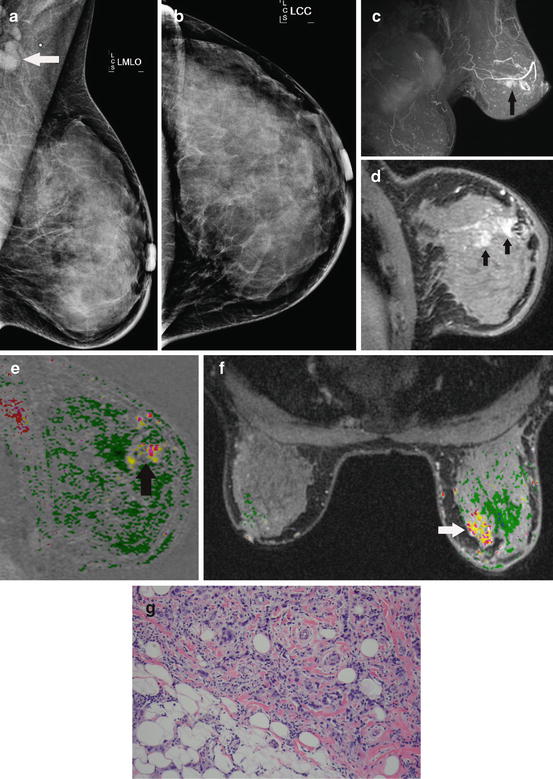

Fig. 8.1
Radiographically dense breasts and axillary adenopathy. This 45-year-old woman presented with a left axillary mass. Workup with diagnostic mammography and ultrasound fail to show any primary. The MLO (a) and CC (b) mammographic views show the enlarged axillary lymph node (arrow) but no abnormality in the breast. She has radiographically dense breasts that are known to impair mammographic sensitivity. Breast MR demonstrates extensive disease in the left breast. The oblique projection maximum intensity projection (MIP) (c) shows extensive enhancement in the left breast (arrow). The oblique sagittal slice (d) depicts the intensive enhancement extending within the ductal ray (arrows). Washout (red) intermixed with persistent (yellow) dynamics is seen with the area of abnormal enhancement (arrow) on the sagittal (e) and axial (f) subtractions with computer-aided detection (CAD). Biopsy of the MR finding shows a Bloom–Richardson grade III/III infiltrating ductal carcinoma (g)
The poor diagnostic performance of mammography in women with dense breasts has prompted several states to pass laws requiring breast imaging centers to inform women if their mammogram shows increased breast density. Breast density is in itself a risk factor amounting to a cumulative lifetime risk of about 20 % [23]. This observation will likely lead to increased use of breast MR for the screening and diagnostic management of women with dense breasts (Fig. 8.1).
High-Risk Histology
Certain benign histologies such as LCIS, atypical ductal hyperplasia, atypical lobular hyperplasia, and peripheral papillomas are considered markers for and increased risk of malignancy. Needle biopsies that result in high-risk histology present management challenges. Excisional biopsy is often recommended due to the frequency of upgrade to malignancy. The upgrade to malignancy for excisional biopsy after a needle biopsy for LCIS ranges from 14 to 50 % [24]. The cancer upgrades are largely attributed to sampling errors that are reduced when larger volumes of tissue are removed with an excisional biopsy. The upgrade rate typically decreases with use of larger bore needles, but a 38 % upgrade rate has been reported with 9-gauge needles [25]. So larger needles is not the whole solution. Atypia has an upgrade rate to malignancy that is generally less than LCIS (10–35 %).
These high-risk lesions reflect an increased risk of malignancy in all the breast tissue, not just the biopsy site. One paper states that the cancer is three times more likely to develop in the ipsilateral breast [26]. Other papers show a preference for the contralateral breast [27]. Three studies have shown a benefit for breast MR screening in patients with high-risk histology (Table 8.2) [27–29]. The cancer detection rate of 3.8–4 % is consistent with the detection rates in the high-risk screening trials. One screening trial only included BRCA patients resulting in about a 9.3 % yield [14]. Most of the other trials were less than the 4 % yield for the high-risk histology trials [2, 7]. The higher biopsy rates for high-risk histology are attributed to the tendency for these patients to have more substantial background enhancement. The American Cancer Society guidelines discussed the potential for including high-risk histology as an indication but cited a lack of clinical trial data [7]. The more recent NCCN guidelines include an indication for breast MR for LCIS [6]. A breast MR will likely be beneficial in the pre-surgical evaluation of women with high-risk histology on needle biopsy prior to surgical excision. The MR may identify occult ipsilateral and contralateral disease that could be missed on excision (Fig. 8.2).
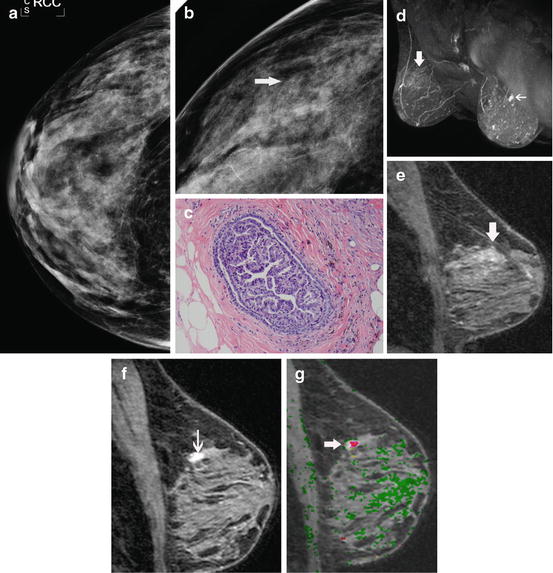
Table 8.2
MR screening in women with high-risk histology
Study | Cancer rate | PPV | Biopsy rate |
|---|---|---|---|
Port (LCIS, ADH) | 4 % (5/135) | 9.2 % (5/46) | 25 % (46/182) |
Friedlander (LCIS) | 3.8 % (5/133) | 18.5 % (5/27) | 18.8 % (25/133) |
Sung (LCIS) | 4 % (10/220) | 20 % (12/60) | 27 % (59/220) |
High-risk genetics studies | 1.1–9.3 % | 7.1–63 % | 2.9–15.8 % |

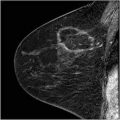
Fig. 8.2
Occult infiltrating carcinoma in a patient with a recent needle biopsy showing atypical ductal hyperplasia. This 59-year-old woman was recalled from screening mammography for a cluster of heterogeneous calcifications in the right breast. The CC (a) and magnification CC (b) mammographic views show the calcifications (arrow). No abnormality was seen in the left breast. A stereotactic biopsy was performed which showed atypical ductal hyperplasia (c). As our routine for all patients with recently diagnosed high-risk histology, a breast MR was performed to evaluate for possible occult disease. Breast MR shows no occult disease in the right breast, but does show a spiculated mass in the left breast that could not be seen on mammography or ultrasound even in retrospect. The MIP of the immediate post-contrast series (d) shows low-level diffuse enhancement from the biopsy site on the right (large arrow) and an intensely enhancing mass (small arrow) on the left. The reformatted sagittal image (e) of the biopsy site on the right shows low-level enhancement typical of post-biopsy change, but no evidence of malignancy. The reformatted sagittal image (f) of the left breast shows intense irregular enhancement typical of infiltrating carcinoma. Washout (red) is depicted on the sagittal subtraction with computer-aided detection (g). Biopsy on the left revealed an infiltrating ductal carcinoma
New MR techniques are particularly well suited for the accurate diagnosis and treatment of intraductal papillomas. Intraductal papilloma is the most common neoplasm associated with unilateral, spontaneous bloody or serous nipple discharge (31–78 %). Yet, malignancy may be seen in 5–18 % of cases [30]. High-contrast, high-resolution breast MR using positive- and negative-scale subtractions provide excellent definition of intraductal lesions, but can also depict malignancies that may be missed with conventional imaging, including galactography (Fig. 8.3). This improved definition may facilitate the correct identification and biopsy of malignancies. Solitary intraductal papillomas are not commonly associated with malignancy. Lesions that have a characteristic appearance of an intraductal papilloma on MR may be removed in their entirety with vacuum-assisted biopsy, sparing unnecessary excision [31, 32].
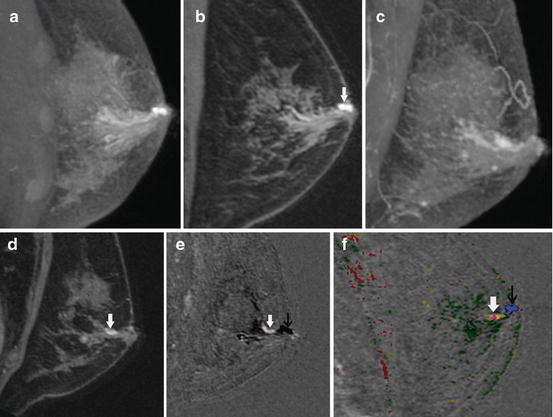

Fig. 8.3
Intraductal papilloma shown with positive- and negative-scale subtracted MR imaging. This 57-year-old patient presented with a spontaneous, unilateral, bloody nipple discharge on the left. The sagittal, pre-contrast, non-spoiled (T2 weighted) maximum intensity projection (MIP) image (a) shows dilated ducts in the subareolar region. A 700-μm oblique slice calculated along the duct from the same pre-contrast acquisition (b) shows hyperintense fluid in a dilated duct (arrow). The sagittal, post-contrast, spoiled (T2 weighting reduced) MIP image (c) shows continued increased signal from dilated ducts due to hemorrhagic fluid shortening the T1. The intraductal mass is not well seen. The 700-μm slice along the duct from the post-contrast acquisition (d) shows a small enhancing lesion (arrow). The relationship to the ducts, however, is not clear. When the pre-contrast, non-spoiled images are subtracted from the post-contrast, spoiled images, the positive- and negative-scale subtracted images (e) provide optimal contrast between enhancing lesions (bright scale) and the fluid-filled ducts (dark scale). The enhancing mass (white arrow) is clearly within the duct (black arrow). This is a typical appearance for an intraductal papilloma. The computer-aided detection (CAD) image (f ) shows a magenta mass depicting plateau enhancement (white arrow) within the dilated duct shown with CAD to be fluid containing, depicted as blue (black arrow). The mass was removed with vacuum-assisted biopsy in its entirety and no surgery was required
Choice of Mastectomy vs. Lumpectomy
Some argue that the use of breast MR needlessly increases the mastectomy rate due to the detection of incidental lesions not seen by conventional imaging [33]. A paper from the Mayo Clinic showed that the breast conservation therapy rate was 54 % in women without MR staging compared with 36 % for those with MR staging. They concluded that MR converted some patients to mastectomy. The women, however, were not randomized and it is likely (by the authors’ admission) that the MR cohort had some additional clinical features such as dense breasts, young age, and positive pathologic margins among others that lead to the MR examination. The non-MR cohort had a higher mastectomy rate than historical controls indicating that there is an overall shift to mastectomy from breast conservation [34].
Bleicher reported similar findings with the mastectomy rate increasing by 1.8 when controlled for T stage. However, there is strong evidence for selection bias in this trial with 130/577 patients having been referred for breast MR. One presumes that the breast MR group was selected for a reason that may predispose to a decision towards mastectomy such as young age, dense breasts, and positive margins. One-third of the MR patients had already undergone one excision before the study. It was not mentioned how many of this group had positive margins. The MR group was younger than the non-MR group. The greater prevalence of dense breasts in younger women that may impair diagnosis on MR due to the more likely presence of signifificant background enhancement [35] .
The biggest problem with most of the breast conservation to mastectomy measurements is that they assume that the conversion is only one way (Fig. 8.4). How can you measure the total effect on management without looking at the potential for conversion from mastectomy to lumpectomy? Hollingsworth evaluated this effect and found that indeed some women were converted to mastectomy, but even more were converted the other way from mastectomy to breast conservation. The net effect was that more patients were having breast conservation (60 %) when compared to historical controls before breast MR (48 %). If the women who elect to have mastectomy but qualified for lumpectomy based upon MR extent are excluded, the breast conservation rate increased to 65 %. Hollingsworth concludes that some women who were leaning towards a choice for mastectomy are in fact reassured by the breast MR and elect for breast conservation. To substantiate this impression, the women who had falsely positive MR (false-positive rate 8.4 %) and subsequently had a benign biopsy had an even higher breast conservation rate (70 %) than the study as a whole (60 %) [36]. A similar paper from Mann et al. that evaluated MR staging for lobular carcinoma showed a nonsignificant trend towards conversion from mastectomy to breast conservation in patients who had a breast MR examination [37]. An example of an MR examination where the improved demonstration of disease extent improved surgical management and probably sparing the patient multiple surgeries or mastectomy is shown in Fig. 8.4.
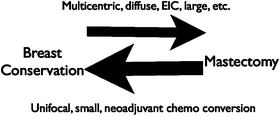

Fig. 8.4
The surgery choice is a two-way street. A number of factors affect the decision for surgical treatment. Information that may affect the decision in favor of mastectomy includes multicentric or diffuse disease, extensive intraductal component, a large tumor size, etc. In some cases, the appearance of the image may favor breast-conserving surgery such as unifocal disease and small size. The addition of neoadjuvant chemotherapy may change an appearance that favors mastectomy towards more localized disease on the post-chemotherapy image. The problem with many of the papers reporting a conversion of treatment decision to mastectomy after MR is that only the situation depicted by the top arrow is considered. Some have reported that if both sides of the street are considered, that MR can result in a net gain for breast conservation
In some cases, where mastectomy is indicated on the basis of clinical or conventional imaging information, MR provides additional information that may alter the mastectomy, convert to patient to breast-conserving surgery, or modify adjuvant therapy. More extensive disease on MR does not necessarily lead to mastectomy. A common situation in our practice is a patient who on the basis of MR extent may be treated with neoadjuvant chemotherapy (Fig. 8.5). MR after neoadjuvant chemotherapy can demonstrate a small residual that could be effectively treated with breast conservation. The mastectomy itself may be modified by the presence of chest wall disease, nipple involvement, and/or skin involvement (Fig. 8.6). MR is very effective in the diagnosis of internal mammary nodal disease. Routine whole breast and partial breast irradiation does not typically treat internal mammary nodes. Radiation therapy may be modified based upon the MR information to include the internal mammary nodes.
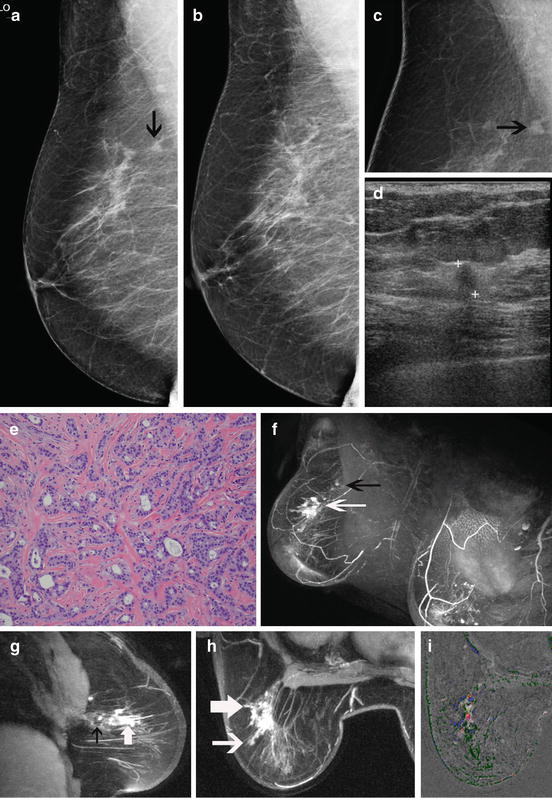
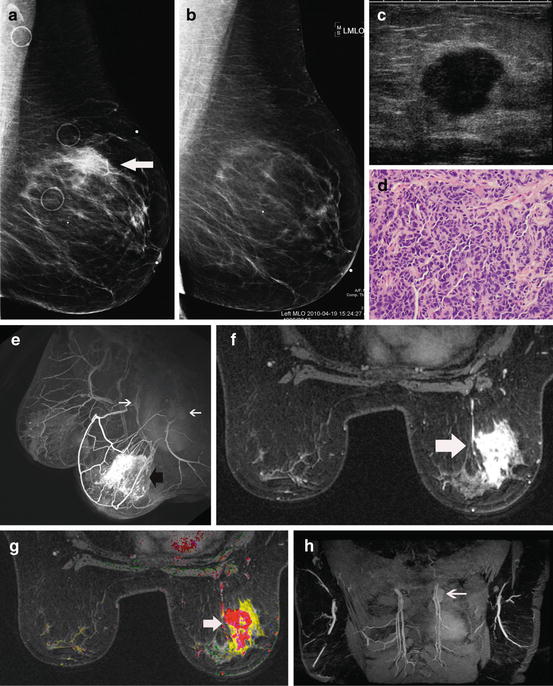
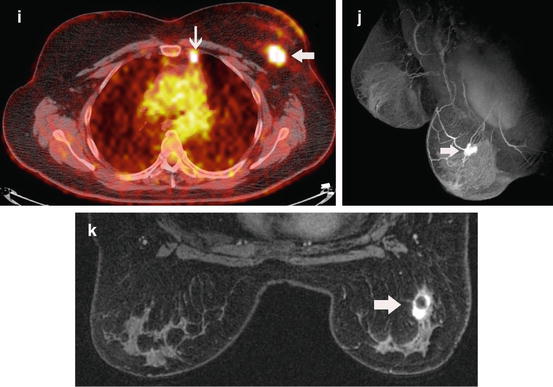

Fig. 8.5
Additional disease seen on MR affects surgery. This 65-year-old patient was recalled from screening mammography for a new irregular mass seen at the 10 o’clock position in the right breast. A small mass (arrow) is shown on the current MLO view (a). The MLO mammographic view (b) from the year before shows no mass. The irregular mass (arrow) is better seen on the magnification view (c). The ultrasound (d) shows a focal hypoechoic 7 mm mass with posterior Fig. 8.5 (continued) acoustic shadowing that is highly suspicious for malignancy. No other lesions could be seen in either breast with conventional imaging. A vacuum-assisted biopsy was obtained showing Bloom–Richardson grade I/III infiltrating ductal carcinoma (e). This would be an example that some might say could be treated with lumpectomy and spared the need for a breast MR. However, the MR shows more extensive non-mass-like enhancement more typical of DCIS. The post-contrast MIP (f) shows a large area of non-mass-like enhancement (white arrow) anterior to the biopsy site (dark arrow) within the same ductal ray. This is better seen on the sagittal oblique thick slice (g) calculated from the immediate post-contrast series. The dark plus-shaped area (black arrow) identifies the artifact from the clip marker at the biopsy site. Non-mass-like enhancement representing occult DCIS extends in the same ductal ray anterior to the biopsy site (white arrow). The axial oblique slice (h) calculated from the post-contrast series shows the extent of the occult DCIS (large arrow). A small projection can be seen extending towards the skin (small arrow). Washout (red) is identified within the mass on the axial subtracted image with computer-aided detection (i). A small lumpectomy would likely have resulted in positive pathologic margins requiring either re-excision or mastectomy. The inability to achieve clear margins may have led to conversion to mastectomy. Using the MR information, the initial surgery was modified so that clear margins could be achieved without the need for re-excision or mastectomy


Fig. 8.6
MR findings alter treatment. This 52-year-old woman was recalled from screening mammography for an asymmetric density in the superior left breast. The MLO mammographic view (a) shows the density in the superior left breast that has developed since the prior study a year before (b). The ultrasound (c) performed at an outside institution was originally thought to be cystic. A cyst aspiration was attempted but failed. Subsequent core needle biopsy showed a Bloom–Richardson grade II/III infiltrating ductal carcinoma (d). Based upon the mammograms and ultrasound, a lumpectomy might have been attempted. The MR information significantly changed management. The oblique projection (e) generated from the immediate post-contrast series shows a large, enhancing mass with spiculated and irregular margins (dark arrow). Also on the projection notice the enlarged axillary and internal mammary (white arrows) nodes. The axial immediate post-contrast image (f) shows the spiculation of the mass (arrow) highly indicative of an infiltrating carcinoma. The axial CAD image (g) shows a predominate mass with washout (red). Washout is associated with highly malignant lesions. The surrounding yellow areas (persistent enhancement) indicate spread of disease into the surrounding tissue. The highly suspicious internal mammary node (arrow) is well demonstrated on the oblique, thick slice image (h) calculated from the immediate post-contrast series. This was confirmed on a PET-CT (i) where the mass (large arrow) and the internal mammary nodal metastasis (small arrow) are shown. If a lumpectomy had been attempted, it is likely that positive margins would have resulted. Re-excision or mastectomy likely would have resulted. Based upon the MR information, the patient had neo-adjuvant chemotherapy. The projection from the immediate post-contrast series (j) performed after chemotherapy shows a concentric response to chemotherapy. Only a small residual remains (arrow). This is better seen on the axial image from the immediate post-contrast series (k). On this image the central low-signal area within the mass (arrow) represents the clip marker surrounded by enhancing residual disease. Based upon the MR information, the patient was able to have successful breast-conserving surgery without the need for re-excision. The radiation therapy was modified to include the internal mammary nodes
Reducing Positive Margins in Breast Conservation Candidates
It is recognized that some women who opt for breast conservation therapy may ultimately be converted to mastectomy due to positive pathologic margins. Huston advocates taking additional margins to reduce the need to re-excision. Taking 4–6 additional margins reduced the re-excision rate from 38.7 to 17.7 % [38]. Re-excision of margins is associated with an increased potential for recurrence. Menes showed the recurrence rate for a single excision to be 4 %, but for 1 and 2 re-excisions the recurrence rate was 7 and 17 %, respectively [39]. O’Sullivan showed a 10-year recurrence rate of 5.6 % if the lumpectomy had clear margins on the first excision, but the recurrence rate increased to 10 % if 2 or more excisions were needed to establish clear margins. The average re-excision rate in the study was 60 % [40]. A large study of 2,206 women reported a single re-excision rate of 22.9 %, two re-excisions 9.4 %, 1.4 % three re-excisions, and ultimate conversions to mastectomy 8.5 % [41]. Unsurprisingly, this is about the same number of conversions to mastectomy with MR information (Fig. 8.7). MR may identify the patients who will likely fail breast conservation surgery due to positive pathologic margins and avoid the unnecessary attempted lumpectomy procedures (Fig. 8.8).
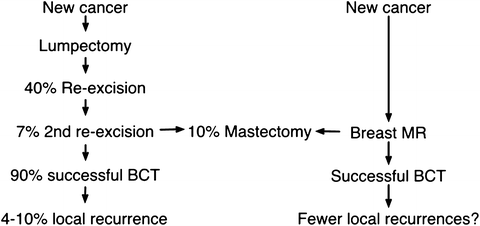
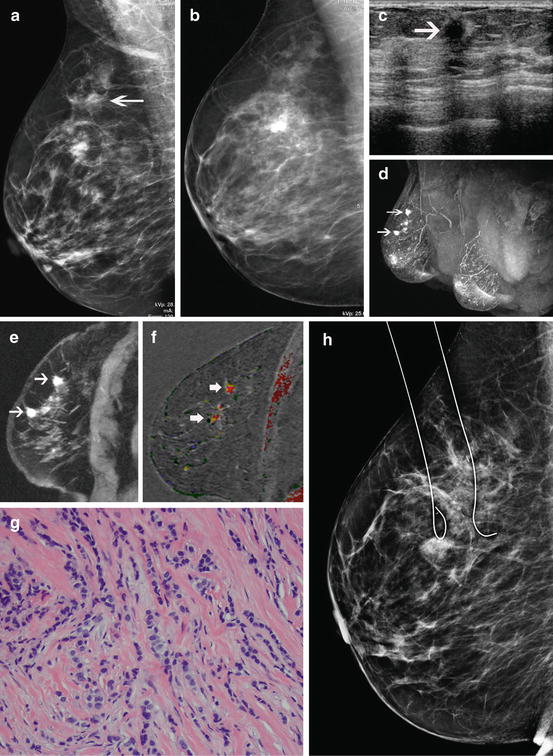

Fig. 8.7
MR may reduce positive margins and identify patients better suited to mastectomy before surgery. Breast conservation candidates may need one or two re-excisions in order to establish clear margins. About 10 % ultimately fail all attempts for clear margins and require mastectomy. MR has two potential benefits: the reduction of positive pathologic margins and the upfront identification of patients best suited for mastectomy. Ultimately, better staging should lead to lower recurrences, but this has not been scientifically established to date

Fig. 8.8
Multifocal lobular carcinoma. This 68-year-old woman was recalled from screening mammography for a subtle increasing focal asymmetry (arrow) in the right breast (a) when compared with the screening mammogram the year before (b). Ultrasound (c) confirmed a hypoechoic mass (arrow) suspicious for malignancy. Because of the subtle findings on mammography, a breast MR was performed which showed additional occult lesions within the same ductal ray. These are well seen on the MIP (d) and the thick oblique sagittal slice (e) both calculated from the immediate post-contrast series. The abnormal enhancement extends over a 6 cm length (arrows) along a ductal ray. The sagittal subtraction with CAD (f) displays washout enhancement (red) within the lesions (arrows). Biopsy reveals lobular carcinoma (g). The patient desired breast-conserving surgery. Since the disease was confined to a long, but narrow ray of tissue, a tailored lumpectomy was performed with MR guidance (h). A cigar-shaped piece of tissue was removed that had clear pathologic margins. Without MR, this patient would likely have had multiple re-excisions in an attempt to get clear margins. Because of the disease extent and lobular histology, this patient likely would have ended her surgical treatment with a mastectomy
Clearly, the establishment of clear margins on the first excision is the goal. However, the rate of positive pathologic margins is high. An acceptable positive margin rate is 25–40 %. Morrow’s group reported a positive margin rate from 1987 to 2006 that varied from 67 to 50 % per year. The ability to establish clear margins did not seem to vary over time. Since the studies were limited to women who had successful breast conservation, the number of women who were ultimately converted to mastectomy is not included. The real positive margin rate was likely much higher [40].
There are other ill effects from positive margins besides the higher recurrence rate. Additional surgeries contribute higher health care costs and morbidity. The location of the excisions may impair the cosmetic result should the patient ultimately opt for breast reconstruction after mastectomy. Decisions regarding adjuvant therapy may be compromised due to the incorrect assessment of tumor size.
Can the improved sensitivity of breast MR be used to reduce positive pathologic margins? Or will the high false-positive rate of breast MR outweigh the possible benefits? There have been two randomized trials that sought to answer if MR could reduce the positive margin rate breast conservation candidates: the COMICE trial in the UK and the MONET trial in the Netherlands [42, 43]. Both of these studies showed that MR provided no benefit.
The COMICE trial was designed to reduce the re-operative rate to below 10 % and was limited to patients scheduled for a wide local excision. Of the 5,496 patients that were eligible, only 1,623 (816 with MR and 807 without MR) were included in the analysis. The re-excision rate for the MR group was 16.3 and 18.7 % for the group without MR (no statistical difference) although they reported a higher mastectomy rate in the MR group (7 %) compared to the control group that was the only possible outcome. Since mastectomy candidates were not evaluated, the conversion of mastectomy to breast conservation by MR could not be observed. Subsequent analysis of the mastectomy specimens demonstrated that in 5 % of the patients mastectomies were justified by demonstration of malignancy outside the lumpectomy site. Biopsy of the incidental MR lesions was not required. Only three multicentric lesions were subjected to biopsy. Only routine pathology analysis of the mastectomy specimens was preformed. So, it is likely that the 2 % “false positives” may have been pathology-missed lesions rather than MR overcalls [42].
The study design emphasized a generalizable (average user) approach rather than optimized to centers of excellence. The ability of a surgeon to exploit the information gleaned from a breast MR takes experience. Yet it appears that most of the surgeons lacked experience in not only breast MR but also breast surgery. 107 surgeons at 45 sites participated in the COMICE study. The 86 % of surgeons that were deemed “high accrual” performed an average of only two or more breast surgeries per year between the years of 2002 and 2007. The adequacy of the margin was left to the surgeon. Probably the biggest criticism of the COMICE study is the lack of quality MR images. The study protocol used 4 mm section thickness coronal slices with no fat suppression. This protocol would be insufficient to pass accreditation and would not even meet the first ACR guidelines issued over a decade ago. The lack of any demonstrated benefit in this study may not mean that breast MR could not succeed in another setting.
The MONET trial in the Netherlands also examined the potential for MR to reduce positive margins with a randomized trial of 418 patients (207 with MR and 211 without). There was no difference in the breast conservation rate or mastectomy rates between the two groups. This study showed a significantly increased re-excision rate of 34 % in the MR group compared to 21 % in the group without MR. It would appear that imaging protocol was more modern than the COMICE. The instrument was a 3 T Philips using dynamic 2 mm section thickness with fat suppression. However, the performance of the breast MR raises some concern. About half the cancers in this study were non-palpable DCIS. Yet, the reported MR sensitivity was only 51 % for DCIS. This is remarkably low compared with other MR trials. The NCI 6883 showed sensitivity for DCIS of 73 %. More modern methods should have sensitivity for DCIS of 80–95 %. The inability to accurately define the margins of DCIS could account for the problems in establishing clear margins in the MR cohort [43].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree