Fig. 27.1
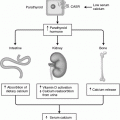
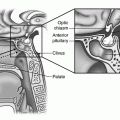
Gross anatomic developmental embryology
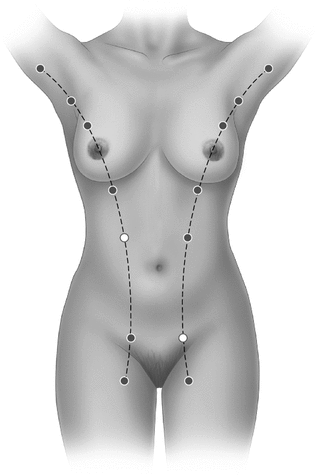
Fig. 27.2
Sites of accessory breast tissue along the embryonic milk line
Two theories of embryogenesis have arisen based on the development of the breast. One is based on the vertebrate evolution sequence and the other on atavism. Since development generally follows the vertebrate evolution sequence and mammary glands have no precursors in reptilian predecessors, it is surprising that they appear so early in embryonic life [1]. However, atavism, the spontaneous reappearance of ancestral characteristics within a species, reminds us that important genetic and developmental information has not been lost during evolution but lies quiescent within the genome and in the process of embryonic development. Atavisms demonstrate that embryologic processes are so complex and integrated that the loss of simple information like the genetic programming for limbs would mean the loss of many other important functions at the same time. The most well-known spontaneous atavisms found in natural populations are the hind limbs in whales and extra toes in horses. An example of an atavism in humans is the appearance of supernumerary nipples, which will be discussed in the section on congenital anomalies below. Abnormal expression of Hox genes has been implicated in the production of atavisms, because of their effect on regulating spatial relationships and the development of structures out of time or out of place [5].
Congenital Anomalies
There are multiple abnormalities of the breast that arise from faulty embryologic mechanisms. These may involve and affect the development of the nipple, the mammary glandular tissue, or the entire breast. Multiple classification systems exist that group the types of defect according to the component of the breast that is affected or the location of these components. However, we agree with Elwood [6] that the precise description of the anatomic defect is more helpful and direct.
Congenital inversion of the nipple is a condition in which mesenchymal infiltration and proliferation beneath the mammary pit does not occur, leading to flat or inverted nipples. This may occur unilaterally or bilaterally having significant functional and aesthetic consequences. Its frequency has been estimated as 17.7 per 1000 women [7], although in a Korean series its prevalence was found to be 3.26% [8]. The majority of congenital inverted nipples can be classified as umbilicated [8, 9] and the rest as invaginated. An important clinical point is that congenital inverted nipples must be differentiated from instances of secondary or acquired nipple inversion, which may due to carcinoma, chronic ductal mastitis, macromastia, and post-breast reduction surgery [10]. Several reconstruction techniques have been described to correct inverted nipples. These range from creating tightness at the neck of the inverted nipple, to dividing the lactiferous ducts and adding soft tissue bulk, to duct-preserving operations with release of fibrosis and placement of purse string sutures [9, 10], and more recently, to a contemporary technique based on the principle of tissue expansion involving body jewelry [11].
Athelia is defined as the absence of the nipple-areola complex. It almost invariably occurs in conjunction with absence of glandular tissue, which together result in complete absence of the breast. Amastia or complete absence of the breast is a rare condition defined by the absence of one or both breasts and nipples. This results from failure of the mammary ridge to develop at 6 weeks of gestation. It is important to note that this condition features different patterns of familial inheritance and may be associated with other anomalies. The original classification dates back to 1965 by Trier, who reported on a series of 43 patients with congenital absence of the breast and classified them according to mode of inheritance and association with anomalies [12]. Since then, only six other cases have been reported in the literature [13, 14]. When this defect is unilateral and occurs in association with absence of pectoral muscles, it is known as Poland’s syndrome. Bilateral congenital absence of the breast in association with ectodermal dysplasia is transmitted in a sex-linked recessive pattern, thus only affecting boys. Other patterns of inheritance include autosomal dominant and autosomal recessive [14].
Athelia may occur in the presence of breast tissue (isolated athelia), very rarely at the normal anatomic site but not infrequently at other accessory locations [1]. A review by Ishida, et al. [15] revealed that ectodermal dysplasia, a hereditary disease of the skin and its appendages, underscores the majority of reports of athelia in the literature. In their case report of isolated athelia, the authors make reference to deficient parathyroid hormone-related protein production as a potential causative factor. This molecule was shown to induce mammary mesenchymal cell formation, triggering morphogenesis of the nipple epidermis [16]. Athelia may also be one of the clinical features in Al-Awadi/Raas-Rothschild syndrome, choanal atresia-athelia syndrome and scalp–ear–nipple syndrome [15]. Historically, the labia minora skin graft was proposed for nipple reconstruction [12], however tattooing of the hypochromic skin is a more recently used and less deforming alternative.
Polythelia is a common abnormality of breast development in which extra-thoracic areas of the mammary ridge do not regress, leading to the formation of supernumerary nipples. These remnants can also develop into complete mammary glands, a condition known as polymastia. This occurs most frequently along the milk line in the axilla, followed by the submammary location. They may also be found in the groin area. However, migration of the embryonic rests [17] may account for polythelia or polymastia found in ectopic locations beyond the mammary ridge, such as the perineum [18], the vulva [19], the posterior thigh [20], and the neck [21]. The prevalence of polythelia ranges from 0.22 to 5%, varying considerably among different ethnic groups. In white European children, polythelia was least prevalent at 0.22% [22], in Black neonates, it was noted to be 1.63% [23], and in Jewish neonates 2.5% [24]. The results of a study by Jaber in 1988 [25] demonstrated a higher prevalence of supernumerary nipples in Arab children—4.7% in infants and 5% in children. This finding was attributed to differences in ethnicity, as well as to high rates of consanguinity in many of the participating families. Other interesting findings in this ethnic group included high incidence of left-side location and positioning just below the normally situated nipple, as well as same sex distribution. A large, early case series dated 1879 by Bruce reported a prevalence of 1.54% among a population of 3956 patients in London [26]. He found that supernumerary nipples occurred more than four times as frequently in men as in women. In addition, there was a remarkable preponderance of supernumerary nipples on the left side. As for the location of supernumerary nipples, the majority of the cases were found on the front of the trunk just below the normal breast near the midline. In a study by Schmidt [27], the prevalence of supernumerary nipples was reported as 5.6% in a series of 502 Caucasian infants. In this series, the higher prevalence may be explained by the fact that both supernumerary areola and supernumerary areola with nipple were ascertained in this population. In fact, supernumerary areola without nipple predominated in this series, as well as predilection for left side and male gender.
While the majority of instances of polythelia occur in sporadic fashion, familial polythelia also has been documented in sequential generations and in siblings [28]. Most pedigrees are consistent with autosomal dominant inheritance with variable expressivity, although X-linked dominant and recessive modes of inheritance are also known to occur [29]. The occurrence of left-sided pseudomammae (nipple and areola with no glandular tissue) inferomedial to the normal breast along the mammary line may be suggestive of a “hereditary” trait of familial cases of accessory mammary tissue. This finding was reported to occur in 72% of the patients in Urbani’s familial series [29]. By comparison, there is a higher frequency of polythelia (nipple only) in the sporadic forms of the condition.
The association of polythelia with genitourinary anomalies has been the subject of debate for many years and still remains unclear whether such link exists. A broad spectrum of nephrourinary defects has been described, including adult polycystic kidney disease, renal agenesis, cystic renal dysplasia, familial renal cysts, and congenital stenosis of the pyeloureteral junction [29]. In one of the first studies examining this issue, Mehes demonstrated that 40% of patients with polythelia also had renal abnormalities, mostly obstructive, an 800-fold increase beyond what would be expected by chance alone [30].This association was later corroborated in a larger study of 10770 infants and children [31], as well as by other investigators [32, 33]. Nevertheless, others have found no such correlation and have challenged this view [18, 23, 24]. Based on their extensive review of the literature, Leung and others recommend at the minimum, an awareness of the possible urologic implications of polythelia, leading to a detailed family history, complete physical examination, and a screening abdominal ultrasound in patients with a definitive diagnosis of polythelia [18, 29].
Sporadic and familial polythelia may also be associated with malignancies of the genitourinary tract such as kidney, testes, prostate, and urinary bladder [34]. Cohen has advocated the inclusion of polythelia in the group of conditions known as genodermatoses with malignant potential, which are inherited disorders of mucocutaneous features predisposing to internal neoplasms [35]. This should prompt surveillance for polythelia-related urinary anomalies and malignancies in patients with supernumerary nipples.
Anatomy
Gross Structure
The anatomy of the breast and its relationship to underlying chest structures is essential in the management of benign and malignant breast diseases (Fig. 27.3a).
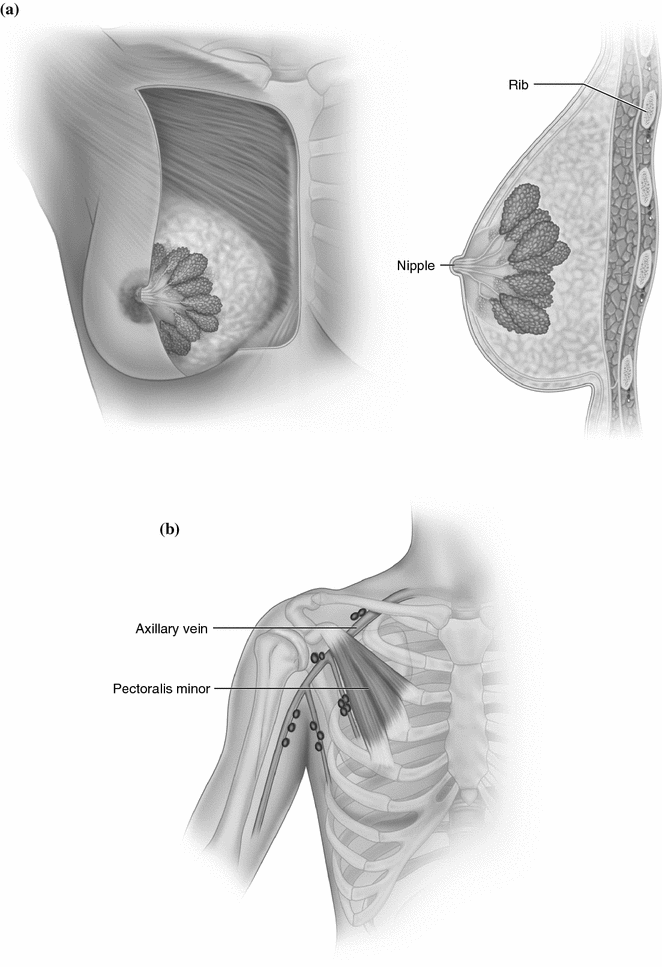

Fig. 27.3
a Structure of breast. b Lymph nodes of the axilla
The mature human breast is a hemispherical eminence located toward the lateral aspect of the pectoral region, encompassing the interval of the third to sixth or seventh ribs, from the side of the sternum to the axilla as the tail of Spence [36]. Its peripheral anatomical boundaries are not precisely defined except in the deep posterior plane, as the gland lies on the pectoralis fascia on a thin layer of loose areolar tissue. Occasionally, microscopic extensions of glandular tissue and posterior suspensory attachments may traverse into the retromammary space and underlying pectoralis fascia. Clinically, infiltration of this space by neoplastic or inflammatory processes may be manifested as fixation of the breast to the chest wall [37]. The retromammary space and deep fascia over the pectoralis muscle are included with the breast in a total mastectomy specimen [38].
The breast parenchyma lies cushioned in fat and invested by the superficial and deep layers of the superficial pectoral fascia. This superficial fascia layer is contiguous with the cervical fascia superiorly and with the superficial abdominal wall fascia inferiorly. Fibrous extensions of the dermis into the glandular tissue of the breast are known as the suspensory ligaments of Cooper, which attach the skin and nipple to the breast. Clinically, distortion of Cooper’s ligaments by parenchymal lesions may result in skin dimpling or nipple retraction [37]. The left breast is generally larger than the right [36]. While minor variations in breast size leading to asymmetry are considered normal and may become more prominent during puberty, breast asymmetry can be visible in 25% of the population [39]. In a series of 500 mammoplasties, severe asymmetry was observed in 5.2% of the cases [40].
The nipple is a conical prominence lined with stratified squamous epithelium and perforated by the multiple openings of the lactiferous ducts. In the prepubertal breast, the nipple is unpigmented. Melanin pigmentation causes the darkening of the nipple and surrounding areola during menarche and most prominently during pregnancy. Various degrees of pigmentation may persist beyond pregnancy and lactation. The glands of Montgomery lie beneath the areola, a specialized ring of mammary skin surrounding the nipple. These modified sebaceous glands exit onto the areola through the tubercles of Montgomery, which become enlarged during pregnancy, and are responsible for secretion of fatty substances that help lubricate the integument of the nipple during lactation [36].
Blood Supply
The arterial blood supply to the breast is derived from the thoracic branches of the axillary, internal mammary, and intercostal arteries [36]. The relative contributions of these vessels vary between individuals and the pattern of circulation may differ between the left and right breasts within an individual. Furthermore, the blood supply of the breast parenchyma does not follow its lobar architecture [41]. In many cases, the perforating branches of the internal mammary artery are the primary perfusion source. These vessels traverse the chest wall near the sternum in the first four intercostal spaces. In 30% of individuals there is no contribution from the axillary artery, while the intercostal arteries are not relevant to the arterial circulation of the breast in nearly 50% of the cases [42]. The venous drainage of the breast is more variable than its arterial supply, but generally follows an anastomotic circle around the base of the nipple, known as the circulus venosus. From there, larger branches disperse through the circumference of the gland and drain into the axillary and internal mammary veins [36].
Lymph Nodes and Nerves
The lymphatic drainage of the breast follows a centrifugal pathway in which lymphatic afferent vessels from the subareolar plexus course along the major lactiferous ducts, retromammary space, and efferent veins into the nodal basins. There are three major mammary lymphatic drainage routes. The principal site of drainage is the axilla, which receives about 75% of lymphatic flow. The central axillary lymph nodes are the major site of drainage, although the external mammary, axillary, scapular, and subclavicular groups are also in the axillary circuit. The internal mammary nodes and interpectoral or Rotter’s nodes constitute the two other pathways of lymph drainage, but are rarely involved exclusively in nodal metastases from breast cancer, except in cases of advanced progression of disease [43]. In addition, there seems to be a differential pattern of lymphatic drainage between palpable and nonpalpable lesions [44].
The loose areolar fat of the axilla contains a variable number of lymph nodes organized into groups. The axillary nodes are divided into three groups according to their position relative to the pectoralis minor muscle (Fig. 27.3b). This division into three levels has standardized the extent of axillary dissection. Level I defines nodes located lateral to the pectoralis minor muscle and includes those in the external mammary, scapular, axillary vein, and central axillary groups. Level II nodes are those that lie beneath the pectoralis minor muscle, which are those in the central axillary group. Level III nodes are located medial to the medial border of the pectoralis minor muscle (subclavicular nodes) and are difficult to visualize and remove unless the muscle is sacrificed as in a standard radical mastectomy [43]. The nerve supply to the breast is derived from the anterior and lateral cutaneous nerves of the fourth to sixth thoracic nerves [45]. However, more important is the knowledge of the nervous structures in the axilla to avoid their injury during axillary lymph node resection. The long thoracic nerve, or external respiratory nerve of Bell, innervates the serratus anterior and arises from the fifth, sixth, and seventh cervical nerves, descends behind the brachial plexus and axillary vein and can be seen coursing close to the chest wall on the medial aspect of the axilla. Its function is important for fixation of the scapula to the chest wall during shoulder adduction and extension of the arm, and its injury results in a winged scapula deformity [38]. The thoracodorsal nerve supplies the latissimus dorsi muscle and arises from the sixth, seventh, and eight cervical nerves making up a branch of the posterior cord of the brachial plexus. It travels with the subscapular artery along the lateral border of the axilla. Its injury results in difficulty with adduction and medial rotation of the arm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree