Outline
Embryology and Development of the Breast
History and Physical Examination
Breast Ultrasonography and Magnetic Resonance Imaging
Fine-Needle Aspiration or Biopsy
Needle Localization and Excision
Image-guided Percutaneous Breast Biopsy
Biologic Markers and Prognostic Factors
Staging of Breast Cancer Using the Tumor–Node–Metastasis System
Key Points
- 1.
Appropriate investigation of symptomatic patients with a breast mass consists of triple assessment and is crucial to rule out an underlying breast malignancy. Triple assessment involves physical examination, breast imaging with mammography and ultrasonography, and pathologic or cytologic evaluation. Nonconcordant results should prompt further investigation or careful follow-up.
- 2.
All women should undergo regular breast screening at the appropriate age, and high-risk patients should be identified for elevated screening pathways dependent on individual risk assessment.
- 3.
Surgical planning needs to be individualized to the patient with regards to suitability for breast conservation versus mastectomy. Adjuvant radiation is an essential treatment component after breast conservation. Reconstructive options should be offered and discussed with all patients undergoing mastectomy.
- 4.
Clinically node-negative patients with invasive cancer should have SLNB for axillary staging. Having two or less positive sentinel nodes in the setting of breast conservation does not require completion axillary dissection. Axillary dissection is required with more extensive nodal positivity or in the setting of any positive sentinel node in patients having mastectomy.
- 5.
BRCA genetic counseling and testing should be offered to women who meet testing criteria. Surveillance programs should include regular breast examination and imaging with mammography and magnetic resonance imaging for those who test positive. Risk-reduction options such as chemoprevention and surgery such as bilateral mastectomies and bilateral salpingo-oophorectomies should be discussed.
Introduction
Breast cancer remains the most common cancer in women and is second only to lung cancer as the leading cause of cancer-related death in the United States. In 2015, an estimated 231,840 new cases of invasive breast cancer were expected to be diagnosed in women in the United States along with 60,290 new cases of noninvasive (in situ) breast cancer. The lifetime risk among women of developing breast cancer is 12.5% (1 in 8); the lifetime risk of dying from breast cancer is 3.6% (1 in 28). About 40,290 women in the United States are expected to die in 2015 from breast cancer, though death rates have been decreasing since 1989. This decline is thought to be secondary to the increased use of mammographic screening with early detection of breast cancer and the use of effective adjuvant therapies. This chapter presents an overview of breast cancer screening, diagnosis, and treatment of benign and malignant conditions of the breast.
Anatomy of the Breast
The adult breast lies between the second and sixth ribs in the vertical plane and between the sternal edge (medially) and midaxillary line (laterally). The average breast measures 10 to 12 cm in diameter and 5 to 7 cm in thickness. It is concentric, with a lateral projection into the axilla named the axillary tail of Spence ( Figs. 14.1 and 14.2 ).
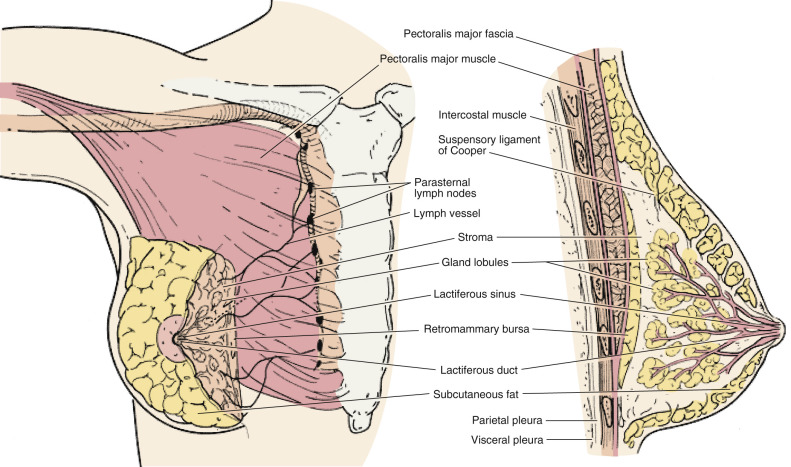
The breast consists of three major structures: skin, subcutaneous fatty tissue, and breast tissue (parenchyma and stroma). The skin contains hair follicles, sebaceous glands, and eccrine sweat glands. The glandular breast is divided into 15 to 20 segments (lobes) that are separated by connective tissue and converge at the nipple in a radial arrangement. These lobes are made up of 20 to 40 lobules, which in turn consist of 10 to 100 alveoli (tubulosaccular secretory units). Five to 10 major milk-collecting ducts drain each segment and open at the nipple into subareolar lactiferous sinuses.
A superficial pectoral fascia envelops the breast; the undersurface of the breast lies on the deep pectoral fascia. The superficial pectoral fascia is continuous with the superficial abdominal fascia of Camper. Between these two fascial layers are fibrous bands called Cooper suspensory ligaments that provide support for the breast. The space between the deep layers of the superficial fascia of the breast and the deep investing fascia of the pectoralis is the retromammary bursa.
The epidermis of the nipple (mammary papilla) and areola is pigmented and wrinkled and consists of keratinized, stratified squamous epithelium that contains smooth muscle fibers in dense connective tissue. These fibers are responsible for the erection of the nipple. Two receptor-type nerve endings (Ruffini-like bodies and end bulb of Krause) are present on the nipple and associated with the tactile reception of stretch and pressure.
The areola has no hair follicles; it has sebaceous glands (at its margin), apocrine sweat glands, and accessory areolar glands (Montgomery glands) that open on the surface of the areola as small elevations called Morgagni tubercles. Montgomery glands are large sebaceous glands capable of secreting milk; they represent an intermediate stage between the sweat and the mammary glands.
The blood supply of the breast is mostly from superficial vessels. The principal blood supply is derived from the internal thoracic (mammary) and lateral thoracic artery and their tributaries. The posterior intercostal arteries of the second to fourth intercostal spaces also give off tributaries called the mammary branches. Approximately 60% of the breast, mainly the medial and central parts, is supplied by the anterior perforating branches of the internal mammary artery. About 30% of the breast, mainly the upper outer quadrant, is supplied by the lateral thoracic artery.
The superficial veins follow the arteries and drain through perforating branches of the internal thoracic vein, tributaries of the axillary vein, and perforating branches of posterior intercostal veins. The veins anastomose circumferentially around the nipple, which is called the circulus venosus.
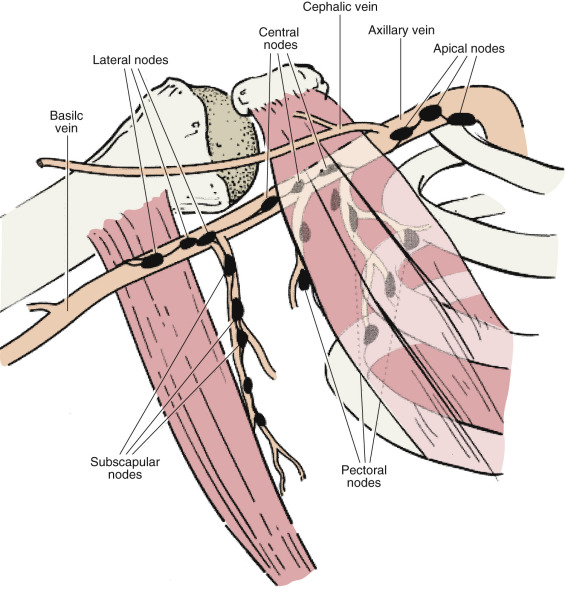
Subepithelial or a papillary plexus of lymphatics of the breast are confluent with the subepithelial lymphatics over the surface of the body. These valveless lymphatic vessels communicate with subdermal lymphatic vessels and merge with Sappey’s subareolar plexus. The subareolar plexus receives lymphatic vessels from the nipple and the areola and communicates by way of the vertical lymphatic vessels that are equivalent to those connecting the subepithelial and subdermal plexus elsewhere in the body. Lymph flows unidirectionally from the superficial to the deep plexus and from the subareolar plexus through the lymphatic vessels of the lactiferous duct to the perilobular and deep subcutaneous plexus. Lymph flow from the deep subcutaneous and intramammary lymphatic vessels moves centrifugally toward the axillary and internal mammary lymph nodes. It is estimated that about 97% of the lymph flows to the axillary nodes and 3% to the internal mammary chain.
Axillary lymph nodes may be divided into three anatomic levels defined in reference to the pectoralis minor muscle. Level I nodes are the axillary vein lymph nodes that lie along the axillary vein from the lateral extent of the pectoralis minor muscle to the latissimus dorsi muscle. In this area, deep to the floor of the axilla, subscapular nodes that lie on the subscapularis muscle are identified.
Level II nodes are designated by their location underneath the pectoralis minor muscle. Medial to the pectoralis minor are level III nodes, which include apical or subclavicular nodes and are adjacent to Halstead’s ligament.
Interpectoral nodes are called Rotter’s nodes. They lie between the pectoralis major and minor muscle.
Embryology and Development of the Breast
The mammary glands are highly specialized skin derivatives of ectodermal origin. The epithelial ridge that develops into breast tissue undergoes a series of proliferations to form the lactiferous ducts. Primitive breast tissue is under the gonadal control of fetal androgen production, which causes a suppression of breast growth during the period of gestation when the tissue is under the simultaneous influence of increasing levels of growth-promoting estrogen and progesterone. After birth, breast tissue remains dormant until adolescence, when estrogen produces a proliferation of ductal epithelium and progesterone produces rapid growth of the acini. However, breast growth and development are not totally dependent on estrogen and progesterone levels. Insulin, cortisol, thyroxine, growth hormone, and prolactin are also required for complete functional development. Minor deficiencies in any one of these hormones can be compensated for by an excess of prolactin.
Pregnancy and Breastfeeding
Increasing amounts of estrogen, progesterone, and human placental lactogen produce active growth of functional breast tissue during the course of pregnancy. Estrogen production is under the control of the fetus. Estrogen influences progesterone production, uteroplacental blood flow, mammary gland development, and fetal adrenal gland function. By the 20th week of pregnancy, most of the estrogen excreted in maternal urine comes from fetal androgens. About 90% of maternal estriol is derived from fetal precursors. Serum prolactin rises from nonpregnant levels of 10 ng/mL to term levels of 200 ng/mL. Amniotic fluid prolactin levels are more than 100 times greater than the levels in maternal or fetal blood early in pregnancy. It is not known whether the fetal pituitary gland or the trophoblast secretes the hormone into the amniotic fluid. Elevated levels of estradiol parallel those of prolactin and indicate that estriol may be responsible for increases in prolactin. Although estrogen may initiate prolactin secretion, high levels block its physiologic effects. Prolactin secretion is also controlled by the prolactin-inhibiting factor. A decrease in estrogen level after delivery and suppression of the prolactin-inhibiting factor by suckling increases prolactin levels. If breastfeeding does not occur, serum prolactin levels decrease to nonpregnant levels in about 1 week.
The release of oxytocin from the posterior pituitary gland affects contraction of the duct system, stimulating the delivery of milk to the nipples. However, after 3 to 4 months of breastfeeding, suckling appears to be the only stimulus required for lactation.
Benign Breast Conditions
Fibrocystic Changes
Fibrocystic change is the most common benign breast condition in women. It consists of no single entity and encompasses a histologic spectrum of changes in the breast, some of which are normal and some of which are abnormal. It is a result of fluctuating hormone levels and is most common in premenopausal women between the ages of 20 and 50 years. It is often associated with pain and tenderness (mastodynia) and tends to be bilateral. Most women report symptoms during the premenstrual phase of the cycle, which suggests that progesterone may play a role in the development and symptoms of cystic alterations in breast tissue. However, the proportional effect of both estrogen and progesterone on the cause of benign breast conditions is unclear.
Fibrocystic change is not a risk factor for cancer. In 1985, the Cancer Committee of the College of American Pathologists published a consensus statement and discouraged the use of the term fibrocystic disease ; they prefer the terms fibrocystic changes or fibrocystic condition.
Mastodynia results from breast stromal edema, ductal dilation, and associated inflammatory response. An increase in breast size is also frequently reported. The differential diagnosis for breast pain includes other conditions affecting the anterior chest wall, such as intercostal neuralgia, myalgia, and chronic costochondritis. Women with large, pendulous breasts will have associated stretching of Cooper ligaments and associated breast pain.
The management of fibrocystic change in any patient presenting symptomatically first consists of ruling out any underlying breast cancer with appropriate imaging and workup. After that, advice can be given with respect to the patient’s breast pain and tenderness. Symptomatic women may benefit from counseling regarding proper selection and fitting of a brassiere because improved mechanical support may relieve breast pain. The effectiveness of dietary interventions to reduce breast pain remains to be established. A low-fat diet was associated with a substantial improvement in mastalgia symptoms when 21 patients with severe mastopathy were randomized to a diet containing 15% fat intake or a general diet containing 36% fat intake. Although many women report that a reduction in caffeine intake alleviates their breast pain, clinical studies have not shown consistent findings. In an uncontrolled study, 61% of women with breast pain who substantially decreased caffeine intake for 1 year had decreased pain or complete relief. Studies with small numbers of patients suggested a potential beneficial effect of vitamin E in fibrocystic change. Evening primrose oil (gammalinolenic acid) has been widely advocated as an option. Two small randomized, double-blind, placebo-controlled studies of evening primrose oil have shown efficacy in the treatment of breast pain. But this result has not been duplicated in other studies.
Hormonal manipulation may alleviate mastodynia but is associated with other side effects. There is no consensus with regards which of these is superior. Danazol is the only medication approved by the US Food and Drug Administration (FDA) for treatment of mastodynia. Overall, 59% to 92% of women treated with danazol experience relief of breast pain. Unfortunately, adverse effects occur in 30% of patients and result in discontinuation of treatment in 15% of patients, even when breast pain is improved. Adverse effects are dose related and are primarily androgenic, including acne, hair loss, decrease in voice pitch, weight gain, headache, nausea, rash, anxiety, and depression.
The selective estrogen receptor modulator (SERM) tamoxifen, which is commonly used to prevent and treat breast cancer, is effective in reducing pain in 71% to 96% of women with cyclic mastalgia and 56% of women with noncyclical mastalgia in controlled trials. In studies comparing tamoxifen dosages and duration for breast pain, the 10 mg/d dosage of tamoxifen was as effective as the 20 mg/d dosage with fewer adverse effects. Tamoxifen has a risk of potentially serious adverse effects, such increased risk of deep vein thrombosis and endometrial cancer. Menopausal symptoms such as hot flashes, nausea, menstrual irregularity, vaginal dryness or discharge, and weight gain have been associated with tamoxifen treatment and often lead to discontinuation.
Proliferative Changes
Proliferative changes include hyperplasia and adenosis. Hyperplasia is proliferation of ductal epithelium, which results in layering of the cells. Atypia may be associated with this proliferation. There are two types of atypical hyperplasia based on microscopic appearance: atypical ductal hyperplasia (ADH) and atypical lobular hyperplasia (ALH). Both are associated with an increased relative risk (RR) of approximately 4 for development of a later invasive breast cancer. Because there is an approximate 25% chance of finding a concurrent cancer with ADH found on core biopsy, surgical excision is recommended. In the case of ALH, recent studies have suggested the invasive cancer “upgrade” rate is lower (0%–6%) after surgical excision of ALH. Therefore, if radiologic findings are concordant or the findings are incidental, excision may not be warranted. All patients with atypical hyperplasia warrant careful clinical and radiologic follow-up.
Adenosis is also a proliferative lesion caused by changes in the acini in the distal mammary lobule. Sclerosing adenosis refers to the dense, fibrotic tissue surrounding these small ducts. These lesions may present as a palpable mass in women in their 30s and 40s.
A papilloma can result from this ductal proliferation. Papillomas are papillary lesions with a branching fibrovascular core surrounded by epithelium. These lesions are associated with serosanguineous nipple discharge in 25% to 50% of presentations. Ninety percent of the time, there is a small palpable mass adjacent to the areola. Intraductal papillomas are rarely associated with carcinoma but require surgical excision to rule out the possibility of misdiagnosis of a malignancy.
Sclerosing Lesions
Sclerosing lesions have been described by a variety of names, including sclerosing papillary proliferation, nonencapsulated sclerosing lesion, indurative mastopathy, and radial scar . They are important because they may simulate carcinoma on mammographic, gross, and microscopic examinations. These lesions are typically less than 1 cm in diameter. On gross examination, they are irregular, gray or white, indurated with central retraction, and have an appearance identical to scirrhous carcinoma. On microscopic examination, the lesion has a stellate configuration and consists of a central, fibrotic core containing entrapped glandular elements. The surrounding breast tissue typically shows varying degrees of intraductal hyperplasia and adenosis. The significance of this lesion relative to subsequent development of carcinoma is controversial. Available evidence suggests that these lesions are part of the fibrocystic complex. It is likely that their premalignant potential is the same as that of the constituent parts. Local excision of these lesions is the treatment of choice.
Fibroadenoma
Fibroadenomas are benign fibroepithelial tumors and are the second most common benign lesion of the breast. They are the most common lesion found in women younger than age 25 years. They persist during the menstrual years of a woman’s life, but regression after menopause has been reported. Patients typically present with a mobile, smooth, painless, palpable mass. Ultrasound examination along with physical examination can help in making the diagnosis.
Mammographically, fibroadenomas may appear as round, oval, or lobulated masses with circumscribed margins. In older women, they can have a rim of coarse calcifications. Fine-needle aspiration (FNA) will reveal benign ductal epithelial cells and elongated dense stromal cells. Microscopically, fibrous tissue comprises most of the fibroadenoma. Carcinoma arising in fibroadenomas is rare.
Fibroadenomas can be followed without the need for complete surgical excision. This can be achieved with physical examination or ultrasound examination if they are not palpable. However, surgical excision should be performed in the following situations:
- •
The mass continues to enlarge.
- •
The results of FNA or core biopsy are inconclusive or yield atypia.
- •
The patient desires surgical excision.
Table 14.1 lists proliferative breast disease and breast cancer risk. Table 14.2 shows breast diagnoses grouped by cancer risk.
| Characteristic | Relative Risk (Confidence Interval) |
|---|---|
| Proliferative disease, no atypia | 1.3 (0.69–11.9) |
| Complex fibroadenoma * | 1.46 (0.53–14.0) |
| Atypical hyperplasia | 2.53 (1.01–6.3) |
| Neither proliferative disease nor complex fibroadenoma | 1.27 (0.89–11.8) |
* Contains cysts, sclerosing adenosis, epithelial calcification, or papillary apocrine changes.
| Cancer Risk | Diagnosis |
|---|---|
| No increased risk | Adenosis, sclerosing or florid Apocrine metaplasia Cysts, macro or micro Duct ectasia Fibroadenoma Fibrosis Hyperplasia (mild) Mastitis Periductal mastitis Squamous metaplasia |
| Slightly increased | Hyperplasia, moderate or florid Papilloma, solid or papillary, with fibrovascular core |
| Moderately increased | Atypical hyperplasia Ductal Lobular |
Phyllodes Tumor
Phyllodes tumors are uncommon, slow-growing fibroepithelial tumors. Previously referred to as cystosarcoma phyllodes, this name contributed to confusion in understanding this entity. Although very similar to a fibroadenoma, the stromal component is hypercellular with increased pleomorphism and mitotic activity. Phyllodes tumors can occur in women of any age but more commonly occur in premenopausal women.
Malignant behavior in phyllodes tumors is rare in premenopausal women. Malignant phyllodes tumors are noted when there is a combination of increased mitotic activity, invasive borders, or marked pleomorphism. Incomplete excision is a major determinant for local recurrence. Treatment is total surgical excision with a wide margin of healthy tissue.
Adenoma
Adenoma of the breast is a well-circumscribed tumor composed of benign epithelial elements with sparse, inconspicuous stroma, a feature that differentiates this lesion from fibroadenoma, in which the stroma is an integral part of the tumor. For practical purposes, adenomas may be divided into two major groups: tubular adenomas and lactating adenomas. Tubular adenomas in young women are well-defined, freely movable nodules that clinically resemble fibroadenomas. Lactating adenomas manifest as one or more freely movable masses during pregnancy or the postpartum period. They are grossly well circumscribed and lobulated; on cut section, they appear tan and softer than tubular adenomas. On microscopic examination, these lesions have lobulated borders and are composed of glands lined by cuboid cells with secretory activity identical to the lactational changes normally observed in the breast tissue during pregnancy and the puerperium.
It should be noted that breast biopsy in a pregnant or lactating woman calls for meticulous hemostasis because of the increased vascularity and risk of postoperative hematoma formation. The lactating breast is predisposed to postoperative infection because milk is a good culture medium. Anesthesia by local injection may be difficult in the enlarged breast but is the method of choice. Incisional biopsy under local anesthesia is an option when excisional biopsy is a problem. Because of the significant risk of infection and milk fistula, the patient who is lactating should cease lactating before biopsy is performed.
Superficial Thrombophlebitis
Superficial thrombophlebitis is also known as Mondor disease of the breast. It is an uncommon benign inflammatory process. It can occur spontaneously but usually is associated with breast trauma, breast surgery, or pregnancy. It is a thrombophlebitis of the thoracoepigastric vein, which drains the upper-outer quadrant of the breast. Patients present with acute pain and a linear, tender fibrotic band with skin retraction over the distribution of the thoracoepigastric vein.
Treatment is conservative, with analgesics and application of heat. The condition resolves in 1 to 3 weeks. Skin retraction superficial to the area of inflammation can remain if the inflammation is extensive. Biopsy is not necessary.
Mastitis
Mastitis usually occurs in relation to lactation. It can occur in nonpuerperal periods in association with galactorrhea. Skin organisms, Staphylococcus aureus, and Streptococcus spp. may cause infection of the nipple and breast ducts. The presence of milk in the ducts can provide an excellent medium for infection.
Women with mastitis may continue to breastfeed. Antibiotic therapy with dicloxacillin sodium (250 mg qid) or penicillin G is indicated. If there is no response, an abscess that may require surgical drainage must be excluded. Inflammatory carcinomas can mimic mastitis, and if no resolution of infection is noted despite continued antibiotics, a skin biopsy may be indicated.
Galactoceles are milk-filled cysts. They are usually tender and present after the abrupt termination of breastfeeding. Aspiration of the cyst is often necessary for symptomatic relief. If reaccumulation occurs, however, surgical excision may be required to avoid infection.
Duct Ectasia
Duct ectasia is a condition that usually occurs in perimenopausal or postmenopausal women. Patients present with a tender, hard, erythematous mass adjacent to the areola in association with burning, itching, or a pulling sensation in the nipple area. A thick, greenish-black discharge may be present. The pathogenesis of this condition has not been fully established. Available evidence suggests, however, that the primary event is periductal inflammation and that ductal ectasia is the ultimate outcome of this disorder. The postulated sequence of events in the evolution of this disease is periductal inflammation leading to periductal fibrosis that subsequently results in ductal dilation. However, the etiology of the initial inflammatory response remains obscure. Histologic evaluation of the area shows dilated, distended terminal-collecting ducts obstructed with inspissated lipid-containing epithelial cells and phagocytic histiocytes. This process tends to occur in a segmental fashion extending from the involved nipple area to adjacent ducts. Occasionally, a small abscess forms at the base of the nipple. Treatment is excisional biopsy.
Younger women can present with inflammation of the ducts in the region of the nipple, which may produce fissures and fistulae with connection from the nipple ducts to the skin at the edges of the areola. Prior periductal mastitis leads to the squamous epithelium of the terminal dilated portion of the collecting ducts to undergo squamous metaplasia. Keratin is formed in the duct, accumulates, and can cause an abscess at the base of the nipple. Excision of the area usually is necessary.
Fat Necrosis
Fat necrosis is a relatively uncommon benign condition occurring as a response to breast trauma or surgery. Patients present with a hard mass that can mimic a carcinoma. The irregular mass is palpable and may involve skin retraction. Multiple calcifications can be seen on mammography.
The histology is active chronic inflammatory cells, with lymphocytes and histiocytes predominating. In the later stages, a collagenous scar is noted, with “oil cysts” or free lipid material released by lipocyte necrosis. Fat necrosis does not increase the risk of carcinoma, and its clinical importance is in the differential diagnosis of a carcinoma.
Nipple Discharge
Nipple discharge has been reported in 10.0% to 15.0% of women with benign breast disease and in 2.5% to 3.0% of those with carcinoma. Galactorrhea presents as bilateral milky nipple discharge consisting of lipid droplets; the condition is usually idiopathic but can be found after discontinuation of oral contraceptives or as a persistent discharge after pregnancy. Plasma prolactin levels should be determined because of the possibility of a prolactin-producing pituitary adenoma.
The discharge is classified according to its appearance as milky, green, bloody, serous, cloudy, or purulent. The drainage should be classified according to whether it is unilateral, bilateral, spontaneous, or recurrent. This information is obtained at the time of a thorough history and physical examination. For example, if the drainage first appeared in the patient’s bra or nightgown on awakening, this finding is significant. The presence of a mass should also be investigated. The risk of cancer is increased when the discharge is unilateral from a single duct, when it occurs in a postmenopausal patient, or when a mass is present.
Unilateral, Spontaneous Nipple Discharge
In cases of unilateral, spontaneous nipple discharge, several causes are included in the differential diagnosis. The most common cause of nipple discharge is mammary-duct ectasia, which produces a multicolored (green, yellow, white, brown, gray, or reddish-brown) nipple discharge. The reddish-brown discharge is often mistaken for a blood discharge. It is thought to result from an increase in glandular secretions, with the production of an irritating lipid fluid that can produce a nipple discharge.
The next most common cause of a multicolored, sticky nipple discharge is nonpuerperal mastitis. The persistent type involves inflammation in deeper portions of the breast; the transient types are associated with periareolar inflammation. If the inflammation develops into an inflammatory mass, surgical excision and drainage are necessary. Medical management with local care, avoidance of all nipple manipulation, and administration of nonsteroidal antiinflammatory agents and an antistaphylococcal antibiotic are often successful when infection is suspected.
Bloody nipple discharge warrants surgical evaluation. Intraductal papillomas are the most common cause of bloody nipple discharge. During the breast examination, physicians should look for an associated periareolar mass. The examination consists of gently and carefully palpating the subareolar region to identify the pressure point that produces the discharge. It is important to reproduce the discharge and demonstrate the breast quadrant from which it emanates. All significant nipple discharges warrant referral for tissue biopsy. Although a mass is usually present when the discharge is a result of cancer, there is no palpable mass in 13% of cancers with nipple secretions. Bloody discharge occurring in the third trimester of pregnancy may be regarded as physiologic, however, and does not require intervention unless persistent for several months after delivery. There are no contraindications to breastfeeding in these patients. Cytology of the discharge is not routinely recommended because of a reported low sensitivity for detection of cancer (≈27%). Galactography (injecting radiopaque contrast into the discharging duct and then performing mammography) offers better visualization of small intraductal papillomas but cannot differentiate between benign and malignant lesions. A surgical procedure is still necessary. Mammography has a 9.5% false-negative rate and a 1.6% false-positive rate for detecting cancer in patients with a nipple discharge. Table 14.3 reveals characteristics of nipple discharge.
| Color | Likely Cause | Percentage Caused by Cancer |
|---|---|---|
| Milky (galactorrhea) | Pituitary adenoma, pregnancy, oral contraceptives | Rare |
| Green, yellow, sticky | Ductal ectasia | Rare |
| Clear, watery | Ductal carcinoma | 30–50 |
| Bloody, sanguineous | Fibrocystic changes, ductal papillomas | 25 |
| Pink, serosanguineous | Fibrocystic changes, ductal papillomas | 10 |
| Yellow, serous | Fibrocystic changes, ductal papillomas | 5 |
| Purulent | Bacterial infection | Rare |
History and Physical Examination
Obtaining a thorough history, including a family history and information on menstrual status, pregnancies and lactation, hormone use, and prior breast surgeries and trauma, is essential. In addition, ascertaining whether the patient performs breast self-examinations (BSEs) and determining the presence and characterization of nipple discharge or a breast mass are important.
Bilateral breast examination is best performed after menstruation and before ovulation. At this time, breast engorgement and tenderness are less likely to be present.
A multipositional breast examination should be performed, including examination in the upright and supine positions. Breast retraction and subtle changes in the skin and nipple may be missed if the patient is examined in only one position.
The patient should be in the sitting position during the inception of the physical breast examination. In this position, asymmetry, skin or nipple retraction, and nipple ulceration should be most apparent ( Fig. 14.3, A ). When the patient’s arms are raised ( Fig. 14.3, B ), skin changes in the lower half of the breast or in the inframammary fold become accentuated. Contraction of the pectoralis major muscle, affected by the patient pushing her hands against her hips ( Fig. 14.3, C ), may demonstrate an otherwise undetected skin retraction. Next, palpation of the breast with the patient still upright may allow detection of subtle lesions that would be more difficult to palpate if she were supine ( Fig. 14.3, D ). Examination of the supraclavicular areas and both sides of the neck for the purpose of detecting suspicious lymphadenopathy is also best done when the patient is in the upright position.
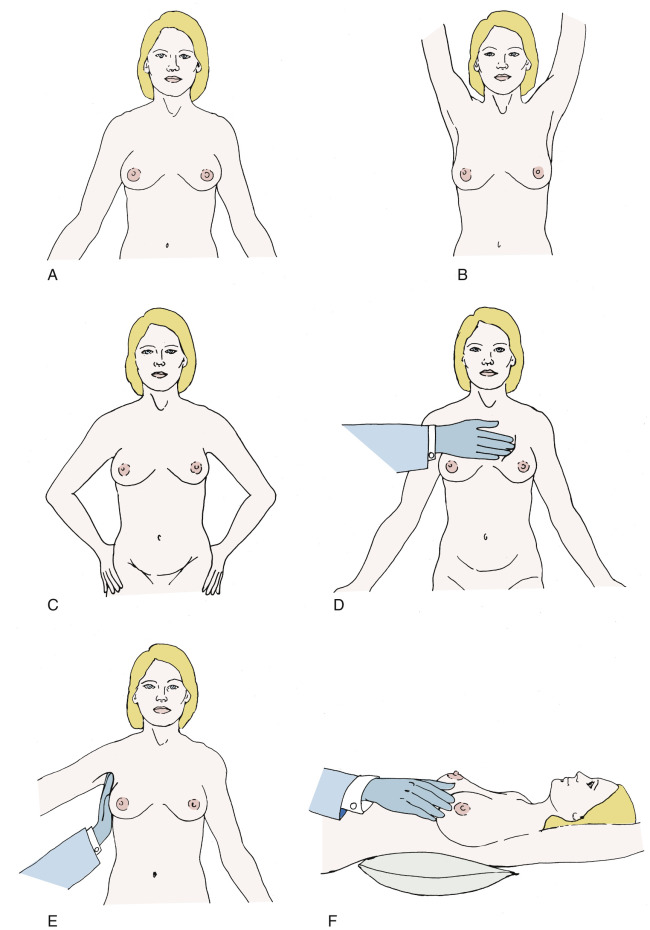
The axilla should be examined with the patient in the upright position. The patient’s right arm should be fixed at the elbow and held there by the physician’s right hand. This allows relaxation of the chest wall musculature ( Fig. 14.3, E ). Palpation with the left hand permits assessment of the lower axilla, and with extension higher toward the clavicle, the middle and upper portions of the axilla can be assessed. The left axilla is examined with the right hand after relaxation of the patient’s left arm in the physician’s left hand. If lymph nodes are palpable, the clinician must assess their level and size and whether they are suspicious, single or multiple, and mobile or fixed to underlying structures.
The second phase of the breast examination is conducted with the patient in the supine position and with the patient’s arm raised above the head ( Fig. 14.3, F ). Digital palpation is carried out using the index and middle fingers and by applying varying amounts of pressure with the flats or pads of the fingers. A thorough examination systemically covers the entire breast and chest wall. The examination can be done in a clockwise direction or by rows (stripwise). It is important to carefully examine beneath the nipple–areolar complex and within the axilla.
An inflammatory appearance of the breast should raise suspicion of an inflammatory carcinoma. The classic appearance of inflammatory breast cancer includes a red, swollen breast with skin edema (“peau d’orange”). The breast is generally not tender. If the inflammation persists after a short course of antibiotics to rule out cellulitis, biopsy of the breast and skin is warranted. Inflammatory breast cancer is often a clinical diagnosis, and a benign skin biopsy should not dissuade the clinician from undertaking further evaluation and treatment. Any asymmetric skin changes or changes of the nipple–areolar complex should arouse suspicion. Paget disease of the nipple suggests the presence of intraductal or invasive cancer involving the nipple, and cancer should be excluded by a nipple biopsy of the abnormal area after a mammogram.
It is important to instruct patients in the technique of BSE. Physician-directed discussion on BSE is the most effective approach. Physicians have the opportunity to reinforce what is normal versus abnormal to patients during the examination. If no abnormal findings are noted on examination, it is critical to document negative findings. The date of the last mammogram, discussion of cancer screening, and plans for follow-up should also be recorded.
Hormones (hormone replacement therapy [HRT] or oral contraceptive pills [OCPs]) should not be renewed without a documented annual breast examination or mammography if indicated. A great deal of litigation results from failure to diagnose breast cancer. The Physician Insurers Association of America’s breast cancer claims study, conducted in 1988, determined that 75% of successful malpractice lawsuits involved primary care physicians with practices in family medicine, internal medicine, or obstetrics and gynecology. It is important that the medical chart include careful documentation because approximately one-third of the cases reported in the Physician Insurers Association of America’s study resulted from inadequate documentation.
Clinical breast examination (CBE) and BSE as methods for screening for breast cancer mainly aim at detection of palpable breast lesions. However, there are no published reports demonstrating these methods as being effective in breast cancer mortality risk reduction.
A Cochrane systematic review published in 2003 included two large population-based studies from Russia and Shanghai, China that compared BSE with no intervention. In both trials, almost twice as many biopsies with benign results were performed in the screening group compared with the control group (RR, 1.89; 95% confidence interval [CI] 1.79–2.00).
Three trials were designed to determine mortality outcomes by using CBE as the primary screening approach in countries with limited health care resources and without mammography screening programs. The applicability of these trials to the United States is limited. One trial in the Philippines was discontinued because of poor acceptance, and the others are ongoing in India and Egypt.
In its most recent update, the United States Preventive Services Task Force (USPSTF) recommended against BSE based on a systematic review of published reports.
For breast cancer screening, the American Cancer Society (ACS) recommends CBE every 3 years for women aged 20 to 39 years and annually beginning at age 40 years.
Mammography
The primary goal of mammography is to screen asymptomatic women to help detect breast cancer at an early stage. The goal of screening mammography is to find cancers before they are clinically palpable, more likely to be small, and less likely to have nodal involvement. Most studies of mammography use mortality as the endpoint, and the value of screening is often limited to mortality rates. Few studies factor the effects of early detection on quality of life or the fact that treatment at earlier stages carries less morbidity and has more treatment options.
In general, a routine screening mammogram consists of a mediolateral oblique (MLO) view and a craniocaudal (CC) view of each breast. With modern low-dose screening, the dose is less than 0.1 rad per study (for comparison, a chest radiograph delivers 0.025 rad per study). The effectiveness of screening depends on the density of the breast: The greater the breast density, the lower the sensitivity of the mammogram.
Breast composition may be one of four patterns of increasing density:
- 1.
Almost entirely fat
- 2.
Scattered fibroglandular densities
- 3.
Heterogeneously dense
- 4.
Extremely dense
It is important to note that the false-negative rate for mammograms is 10% to 15% and that a normal mammogram does not eliminate the need for further evaluation of a dominant mass in the breast. If the clinical examination is suspicious, a negative mammogram result should not delay further investigation. Breast density should be documented in the mammogram report, and many states require notification of the patients of their breast density in their results letter.
Mammographic screening in women 40 years or older has reduced mortality rates by 20% to 30%. The efficacy of screening mammography in decreasing breast cancer mortality rates has been demonstrated in numerous studies. In the 1960s, the Health Insurance Plan of Greater New York performed a study of physical examination and mammography in a study group of 30,756 women and a control group of 30,239 women between the ages of 40 and 64 years. At 10 years of follow-up, the study group had a 30% decrease in the breast cancer mortality rate compared with the control group.
A total of eight large randomized trials on mammographic screening have been conducted. Six of the eight trials revealed a statistically significant reduction in mortality with mammographic screening. The reduction in mortality rate was not as evident among women between the ages of 40 and 49 years compared with women older than 50 years of age. The relative mortality rate reduction appears later in women between the ages of 40 and 49 at randomization compared with women 50 years or older. It is also likely that the small numbers of women between 40 and 49 years of age in the existing randomized trials may have contributed to this difference.
In a meta-analysis of eight randomized controlled trials (RCTs) of mammographic screening, a statistically significant 18% reduction in mortality in women aged 40 to 49 years was noted. Combined data from five Swedish trials yielded a statistically significant mortality decrease of 29% ( Table 14.4 ).
| Trial | Relative Risk (95% Confidence Interval) |
|---|---|
| Malmö | 0.96 (0.68–1.35) |
| Canada | 1.08 (0.84–1.40) |
| Göteborg | 0.55 (0.31–0.95) |
| Stockholm | 0.73 (0.50–1.06) |
| Kopparberg | 0.58 (0.45–0.76) |
| Östergötland | 0.76 (0.61–0.95) |
| New York | 0.79 (0.64–0.98) |
| Edinburgh | 0.87 (0.70–1.08) |
The updated Cochrane systematic review was published in 2013. Because of the inclusion criteria chosen for the review, they reported on six randomized trials. They concluded that screening is likely to reduce breast cancer mortality. The effect was lowest in the adequately randomized trials, and a reasonable estimate is an overall 15% reduction in mortality from breast cancer (RR, 0.81; 95% CI 0.74–0.87) after 13 years. However, it should be noted that there is a false-positive rate associated with screening.
Screening Interval
For several years, there has been a significant debate about the appropriate age at which to commence mammographic screening. Whereas the 2003 ACS guidelines for mammographic screening recommended a yearly mammogram starting at age 40 tears, the National Cancer Institute (NCI) recommended a mammogram every 1 or 2 years. The American College of Obstetricians and Gynecologists (ACOG) currently recommends annual mammography from 40 years of age. In October 2015, the ACS revised its guidelines with regards to screening mammography in average-risk women. The guidelines now advocate the commencement of annual mammography at age 45 years until age 54 years. Thereafter, from age 55 years onward, biennial mammograms are recommended while the patient’s overall health is good or she has a life expectancy of 10 years or longer. The ACS falls short of recommending mammography from age 40 to 44 years but state that women should have the opportunity to commence screening. Table 14.5 lists recommendations by society.
| Age (Years) | American College of Obstetricians and Gynecologists | American Cancer Society | National Cancer Institute | US Preventive Services Task Force |
|---|---|---|---|---|
| 40–45 | Annually | Not recommended but should have opportunity to begin | 1–2 years | Individual decision |
| 45–50 | Annually | Annually | 1–2 years | Individual decision |
| 50–55 | Annually | Annually | 1–2 years | 2 years |
| 55–74 | Annually | 2 years | 1–2 years | 2 years |
Data from the Breast Cancer Surveillance Consortium, also recently published in October 2015, somewhat support this strategy. They reviewed screening mammography from 15,440 patients from ages 40 to 85 years who had either annual or biennial imaging. Premenopausal women diagnosed at the longer interval screening time point had more tumors with less favorable prognostic characteristics—that is, stage IIB or higher—than those screened annually. In those who were postmenopausal and not taking HRT, there was no difference in the proportions of stage IIB or higher tumors whether they were screened at 1 or 2 years. This would suggest that menopausal status rather than age may be a better factor to consider when determining mammographic screening interval. Biologically, this is plausible with the possibility that estrogen-exposed tumors may grow faster and thus have a decreased detectable preclinical phase combined with increased breast density premenopausally decreasing mammographic sensitivity.
The USPSTF published its screening recommendations in 2009 and currently has draft recommendations due for publication in 2015. After reviewing the evidence regarding the efficacy of BSE, CBE, and mammography in reducing breast cancer mortality, it recommends starting mammography at age 50 years and performing biennial screening. It does not recommend routine mammography in women younger than age 50 years but recommends that the decision is an individual one. They advise that, although there may be an improvement in the risk of dying from breast cancer with screening before age 50 years, that the advantage is not as big as in older women. They also note that it is associated with a higher false-positive rate in those younger than age 50 years; therefore, an individual can decide on her personal acceptability of the risk-to-benefit ratio. The task force cites the risk of false-positive mammography results (≤56%) as a potential harm and considers pain, anxiety, distress, and other psychologic responses as potential harms. However, the task force states that these harms are transient and thus do not represent a substantial deterrent to the continued use of mammography for screening. These recommendations have caused much controversy and debate in the medical community and lay press since they were announced in 2009. The majority of clinical societies did not change their recommendations after these guidelines were issued. Annual screening mammography may commence earlier than age 40 in a few special circumstances ( Table 14.6 ).
| Condition | Timing of Annual Mammography |
|---|---|
| Lobular cancer in situ or breast cancer diagnosis | At time of diagnosis |
| First-degree relative with premenopausal breast cancer | 10 years earlier than relative’s age at diagnosis but not younger than 25 years |
| Mantle irradiation for Hodgkin disease | 8 years after completion of radiation therapy |
| BRCA1 or BRCA2 mutation | Age 25–35 years; specific age chosen based on adequacy of mammography imaging in the first study and patient choice |
Breast Imaging Reporting and Data System
In the past, a lack of uniformity in mammography terminology and reporting often led to confusion as to the malignant nature of a lesion. In 1994, the Mammography Quality Standards Act was passed by Congress and is administered by the FDA. It requires that mammography facilities monitor the results of their breast cancer detection programs, including the number of recommended biopsies and the size, number, and stage of cancers detected. The American College of Radiology (ACR) uses a terminology and lexicon system called the Breast Imaging Reporting and Data System (BI-RADS) for reporting abnormalities seen on mammography ( Table 14.7 ). This standardized reporting system was developed in 1995. Each category leads to a fixed assessment and specific management recommendations. In addition, associated findings, such as skin or nipple retraction, skin thickening, skin lesions, axillary adenopathy, and the presence of architectural distortion, should also be reported.
| BI-RADS Category | Assessment |
|---|---|
| ASSESSMENT IS INCOMPLETE | |
| 0 | Need additional imaging evaluation and/or prior mammograms for comparison |
| ASSESSMENT IS COMPLETE: FINAL CATEGORIES | |
| 1 | Negative |
| 2 | Benign finding(s) |
| 3 | Probably benign finding; initial short-interval follow-up suggested |
| 4 * | Suspicious abnormality; biopsy should be considered |
| Optional subdivisions: | |
| 4A: Low suspicion for malignancy | |
| 4B: Moderate suspicion of malignancy | |
| 4C: High suspicion for malignancy | |
| 5 | Highly suggestive of malignancy; appropriate action should be taken |
| 6 | Known biopsy; proven malignancy; appropriate action should be taken |
* By subdividing category 4 into 4A, 4B, and 4C, it is encouraged that relevant probabilities for malignancy be indicated within this category so the patient and her physician can make an informed decision on the ultimate course of action.
The predictors of malignancy for the BI-RADS categories are 0% to 2% for category 3 and approximately 98% or greater for category 5. Category 4 is less predictable. Liberman and colleagues and Orel and colleagues have placed the risk of malignancy for this category at around 30%.
The ACR Task Force has published a fifth edition of the BI-RADS classification system in the form of a BI-RADS Atlas that includes second edition BI-RADS classifications for ultrasonography and MRI. The new edition of BI-RADS includes the previously mentioned classification of patterns of breast density for uniform reporting in mammogram reports. Category 4 is divided into three parts based on the prebiopsy risk for malignancy of the lesion—4A (low suspicion), 4B (moderate), and 4C (high)—in an effort to better guide clinicians and to collect meaningful data about this category. By subdividing the category, the ACR hopes to provide better communication to the referring physician about the prebiopsy risk of malignancy. One recent retrospective study evaluated interobserver variability and positive predictive value of BI-RADS categories 4A, 4B, and 4C. The risk of malignancy was found to be 6%, 15%, and 53%, respectively. Table 14.7 shows BI-RADS reporting.
Diagnostic Mammography
Abnormalities found on mammographic screening may need further evaluation with additional mammography views or other imaging modalities, such as ultrasonography or magnetic resonance imaging (MRI). In some screening programs, the mammograms are reviewed by the radiologist as they are performed, and if additional views are needed, they are performed on the same day. In other programs, if additional studies are required, the patient is called back for them at a later date. In several studies, the frequency of “call-backs” has ranged from 5% to 11%.
Mammographic Lesions
A “mass” is defined as a space-occupying lesion seen in two different projections. If a possible mass is seen on only one view, it is called a “density” until its three dimensionality is confirmed. A description of the shape and the margins of the lesion are also necessary. The highest frequency of carcinoma is noted in masses that have an irregular shape or spiculated borders. These lesions are associated with pleomorphic calcifications that appear discontinuous and linear in distribution. This discontinuous linear pattern suggests irregular filling of a duct with abnormal cells.
Microcalcifications
The BI-RADS lexicon describes calcification morphology (shape) and distribution. Calcifications may be scattered or clustered, coarse or fine, and old or new. Comparison with prior mammograms is often necessary ( Table 14.8 ).
| Morphology | Description and Associated Lesion |
|---|---|
| Typically benign | Includes skin (lucent centered) Vascular (parallel tracks) Coarse “popcorn-like” (fibroadenomas) Large rodlike (secretory disease) Eggshell or rim (fat necrosis) Milk of calcium (within tiny cysts) Dystrophic (after trauma or irradiation) |
| Suspicious | Amorphous Coarse heterogenous Fine pleomorphic Fine linear or fine linear branching |
| Distribution | Diffuse Regional Grouped Linear Segmental |
Digital Mammography
The benefits of digital mammography over traditional film mammography concern image acquisition and facilitation of storage. In addition, digital-image processing allows manipulation of image contrast and may enhance subtle contrast differences. In January 2000, the General Electric Senographe 2000D was approved by the FDA. Since its introduction, population-based screening trials comparing screen-film and full-field digital mammography have been conducted. In the Oslo I Study conducted in Norway, full-field digital and screen-film mammography were performed in 3683 women aged 50 to 69 years. The investigators found no statistically significant difference in cancer detection rates between the two modalities. The Oslo II Study yielded similar results in cancer detection rates. Full-field digital mammography did yield higher cancer detection rates, but this difference between film and digital mammography was not statistically significant. In Canada and the United States, 49,528 women were enrolled in the Digital Mammographic Imaging Screening Trial (DMIST). All participants underwent both digital and film mammography in random order. The investigators noted that, although the diagnostic accuracy of digital and film mammography was similar, the accuracy of digital mammography was better in women younger than age 50 years, women with radiographically dense breasts, and premenopausal or perimenopausal women. Thus, in women who met those criteria, the investigators recommended digital mammography. Digital mammography is now available in most screening centers. It can be also combined with computer-aided detection (CAD), which is a process designed to analyze mammographic images for suspicious areas; it is in effect a “second pair of eyes” for the radiologist. The ACR believes that CAD, when used for screening or diagnostic film screen mammography, can be a valuable procedure to aid in the early detection of breast cancer.
Digital tomosynthesis, or three-dimensional mammography, is approved by the FDA but is not yet considered the standard of care for breast cancer screening. Other experimental methods of breast imaging are being investigated but are not in routine use such as contrast-enhanced digital mammography, which is a recent development of digital mammography using the intravenous injection of an iodinated contrast agent in conjunction with a mammography examination.
Breast Ultrasonography and Magnetic Resonance Imaging
Breast ultrasonography can be used to distinguish between solid and cystic masses in the breast. It can be used to evaluate a focal mass identified on a mammogram or a palpable mass. It is also used as an adjuvant for biopsy. Because of its low specificity, it is not thought to be a good modality for screening. It cannot replace mammography because it has no ability to detect microcalcifications. Ultrasonography can complement mammography in young women with dense breasts because dense breasts limit the accuracy of the mammogram.
Magnetic resonance imaging has a high sensitivity for the detection of breast cancer, and it is not affected by breast density. Screening studies to date have been done in high-risk patients, primarily those at risk because of known or suspected BRCA mutations or a family history of breast cancer. A systematic review by Warner and colleagues in 2008 identified 11 prospective studies of MRI screening. In the systematic review, MRI’s sensitivity was 75%. Combining mammography and MRI increased the sensitivity to 84%. The specificity of MRI (96⋅1%) was comparable but marginally lower than the specificity of mammography (98⋅5%). These studies on the use of MRI for surveillance of women at high risk for hereditary breast cancer have demonstrated a substantial benefit for breast cancer detection.
The ACS has issued guidelines about the use of annual MRI for screening in high-risk women. A guideline panel was assembled to review evidence and develop new recommendations for women at different levels of risk. Based on this panel screening, MRI is recommended for women with an approximately 20% to 25% or greater lifetime risk of developing breast cancer. This includes women with strong family history of breast or ovarian cancer, untested first-degree relatives of BRCA mutation carriers, and women with a history of mantle radiation between ages 10 to 30 years. Screening MRI is also recommended for patients with Li-Fraumeni or Cowden syndrome and their first-degree relatives.
The optimal point in time to perform the MRI in relation to mammography has not currently been determined.
MRI can be useful in treatment selection for newly diagnosed breast cancers, particularly in evaluating tumor multifocality in women wanting to pursue breast conservation. However, it has not been shown to increase the likelihood of negative margins or the need to convert from lumpectomy to mastectomy. It is used to assess for residual disease after neoadjuvant chemotherapy. It is also a useful tool to assess for an occult primary breast tumor in patients presenting with axillary nodal metastases. There is insufficient evidence to recommend for or against screening with MRI for women with a personal history of breast cancer, atypical hyperplasia, or extremely dense breasts on mammography.
Diagnostic Evaluation
Palpable Mass
The workup of a patient with a dominant mass should include bilateral mammography. In addition to gaining valuable information about the characteristics of the mass, a secondary purpose in this setting is to screen the normal surrounding breast and the contralateral breast for nonpalpable mammographic abnormalities (densities or calcifications). Evaluation of a palpable mass is important to determine whether the mass is cancerous even if the mammogram result is negative.
Fine-Needle Aspiration or Biopsy
Fine-needle aspiration can be extremely useful in providing a cytologic analysis of a palpable breast mass. Many palpable thickenings and all dominant masses should be considered for FNA because it can differentiate between solid and cystic masses. In addition, FNA can diagnose and treat simple cysts and provide cellular material for cytologic analysis. The FNA should be performed after radiologic examination because the resultant hematoma could mask an underlying abnormality.
The breast is prepped with alcohol; with the physician facing the patient, the lesion is stabilized with the physician’s opposite hand. Usually, a 21- or 25-gauge needle on a 10-cc syringe is used. Approximately 3 cc of air is aspirated into the syringe to facilitate expulsion of the contents onto the slide after the procedure. The needle is introduced into the lesion, and suction is applied on the syringe. If the mass is cystic, the fluid is completely evacuated, and the lesion should completely disappear. The syringe is withdrawn, and the fluid is discarded if it is serous and nonbloody. The patient should return in 4 to 6 weeks for reexamination.
If the lesion encountered is not cystic or suspected to be solid, an FNA biopsy can be performed in the same manner. After insertion into the lesion, multiple passes (10–15) through the lesion with changes in direction allow extensive sampling and create a “feel” for the mass (carcinomas are usually hard and gritty). The goal of sampling is to obtain material in the hub of the needle, not to fill the syringe. Care should be taken to release the suction before withdrawing the needle to prevent aspiration into the syringe. The sample is then ejected onto a glass slide, gently smeared with another slide, and placed in sterile jars containing 95% ethanol for transport to the cytology laboratory. Alternatively, it can be placed in a specimen jar containing cytofixative. The needle should be removed from the syringe, the medium aspirated into the syringe, the needle replaced, and the medium then ejected into the jar.
An FNA requires a cytopathologist experienced in breast pathology. The false-negative rate can range from 3% to 35% depending on the expertise of the aspirator and cytopathologist, the size of the lesion, the location within the breast, and the cellular composition of the lesion. Negative findings of an FNA sample in the presence of a suspicious mass should not preclude further diagnostic evaluation. A diagnosis of atypical cells after an FNA warrants a surgical biopsy. Any mass remaining after aspiration of a cyst should be excised. Similarly, a cyst that recurs in the same location after one or two aspirations should be excised.
The false-positive rate of an FNA sample is less than 1%, but in the United States most surgeons do not perform definitive surgery such as a mastectomy without a prior surgical biopsy, core-needle biopsy, or frozen-section diagnosis at the time of surgery. An FNA sample that is positive for adenocarcinoma could, however, provides a preliminary diagnosis and guides subsequent management.
Patients with palpable solid masses can have a biopsy of the mass in the office with use of a Tru-Cut 14.gauge biopsy device. The breast is prepped sterilely, and a local anesthetic is used to infiltrate the skin. A small nick is made in the skin with a scalpel to accommodate the biopsy instrument. A core biopsy of the solid mass is obtained. The instrument has a “firing range” and therefore should be kept parallel to the chest wall to avoid penetrating trauma. The specimen is placed in formalin and sent for pathologic examination. It is believed that if the specimen “floats” in the solution, it is likely nondiagnostic fat. Tumor specimens have a grayish appearance and typically “sink” in the solution.
Needle Localization and Excision
Needle localization is a technique that allows surgical excision of a lesion that is nonpalpable. The technique uses a hook and wire system to target the lesion, and image guidance can be provided by mammography; ultrasonography; and, in some cases, MRI. In mammography-guided needle localization, coordinates of the lesion are obtained by placing the breast in an alphanumeric grid. The needle is inserted and, when adequate placement is noted, the hook wire is deployed and the needle removed. Two mammographic views are then obtained.
The mammography films are available intraoperatively and show the relationship between the lesion and localizing hook. Excision with needle localization allows the surgeon to minimize the amount of breast tissue removed by following the needle to the targeted lesion. After removal, a specimen radiograph is obtained to ensure that successful removal of the lesion has been performed. Radiologists and surgeons experienced in needle localization and excisions report only 0.2% to 0.3% of lesions missed with this approach. The specimen radiograph helps to ascertain the lesion was not missed.
Another variation of this approach to excising nonpalpable lesions is the use of a radioactive seed, which involves a small titanium seed (4 × 0.8 mm) that is labeled with iodine-125 and placed in a similar fashion to the needle localization. Use of an intraoperative handheld gamma probe allows for accurate excision on the lesion, which is similarly radiographed to ensure retrieval of the lesion and the radioactive seed. It has advantages over needle localization because the placement of the radioactive can be performed several days before the procedure, which can improve flexibility with regards to scheduling.
Image-guided Percutaneous Breast Biopsy
With the current advancements available in breast imaging, percutaneous image-guided breast biopsy is increasingly being used as an alternative to surgical biopsy. Percutaneous biopsy methods differ with respect to the method of imaging guidance and the tissue-acquisition device used. The use of image-guided percutaneous biopsy has advantages over surgical excision for the diagnosis of breast lesions. It is less invasive, and because less tissue is removed, it will result in less scarring on subsequent mammograms. Regardless of whether the diagnosis is benign or malignant, the patients who have percutaneous biopsies will undergo fewer operations. In addition, in cases of malignancy, the discussion and surgical treatment plan can be streamlined. The choice of which image-guided modality to use depends on the lesion. Stereotactic biopsy is best for calcifications. If a lesion is seen on ultrasonography, it is best to use that modality because it is easier to use and has been reported to be less costly.
Stereotactic Biopsy
Stereotactic-guided core needle biopsy uses specialized mammography equipment to calculate the location of a lesion in three dimensions. Stereotactic biopsy can be performed with the patient prone on a dedicated table or with the patient sitting in an upright unit. Some patients may not be candidates for this approach. A patient may be too large to be accommodated by the system. The thickness of the breast must be adequate to accommodate to the automated biopsy device. Abnormalities just under the skin may also pose technical problems. A vague asymmetric density or diffuse group of widely separated calcifications may present difficulties. Patients who cannot remain prone or are unable to cooperate for the 20- to 40-minute duration of the procedure may not be candidates.
An automated core needle or directional vacuum-assisted biopsy probe is used to obtain the tissue specimens. Multiple tissue specimens are obtained for pathologic analysis. Many reports in the medical literature state the procedure has a sensitivity of 70% to 100% and a specificity of 85% to 100%. Studies have shown 99% accuracy with a 14-gauge needle obtaining five specimens. Radiography should be performed routinely on women with specimens of breast microcalcifications to determine whether calcifications were obtained.
Ultrasound-guided Biopsy
The use of ultrasound imaging for percutaneous biopsy of lesions seen on ultrasonography has certain advantages. For example, it requires no specialized equipment, requires no radiation exposure, and has the ability to sample areas that may be inaccessible with stereotactic biopsy (eg, the axilla). A 14-gauge automated needle is used, and real-time imaging allows accurate positioning. Multiple tissue core samples are sent for pathologic analysis. Consideration to placement of a clip to demarcate the area of biopsy is necessary, particularly in cases in which neoadjuvant chemotherapy will be considered.
Tissue-acquisition Devices
Available tissue-acquisition devices include fine needles, automated core needles, directional vacuum-assisted probes, and biopsy cannulas. Excellent results have been obtained using the 14-gauge automated needle for biopsy of masses under ultrasound or stereotactic guidance. Most centers use larger tissue-acquisition devices instead of fine needles because of accuracy of tissue diagnosis when a larger volume of tissue is obtained. Compared with the automated needle, the vacuum device acquires larger samples of tissue, has a higher frequency of retrieval of calcifications, and may provide more accurate lesion characterization. Accurate placement of a localizing clip through the biopsy probe is necessary to facilitate subsequent localization if needed.
Surgical Excision and Breast Biopsy
A biopsy can be performed on an outpatient basis under local anesthesia in the majority of patients. It is important to choose the appropriate incision and location. Unless the lesion is close to the nipple or suspected to be a fibroadenoma, the incision should be made in close proximity to the mass and not circumareolar. The surgeon should keep in mind the possibility of subsequent mastectomy when placing the incision. Many times, the biopsy is part of the treatment. The specimen should be adequately oriented for margin analysis by the pathologist and also sent for the appropriate markers such as estrogen-receptor (ER) and progesterone-receptor (PR) status and HER2/neu. Orientation of the specimen is important because a reexcision of an involved margin may need to be subsequently performed.
The incision should be closed with fine suture material with a subcuticular closure. Hemostasis needs to be ascertained before closing and is usually achieved with electrocautery. Weck clips can be placed in the cavity bed if a diagnosis of breast cancer is known and breast conservation is planned. No particular immobilization is required, but a good support bra is recommended to minimize hematoma, induration, and discomfort.
Certain benign lesions on core needle biopsy have a relatively high incidence of coexisting carcinoma found on subsequent surgical excision. The small volume of tissue obtained on core needle biopsy in these cases may not be adequate to rule out cancer. Atypical ductal hyperplasia is the most commonly encountered of these lesions. About 25% of atypical ductal hyperplasia on the core biopsy specimen will have carcinoma on excisional biopsy. Radial scars are reported to have a coexistent carcinoma in 20% and therefore also require surgical excision. The tissue obtained from a core needle biopsy may not be concordant with the imaging findings. In a study by Dershaw and colleagues, repeated biopsy for nonconcordance found carcinoma in 47% of cases. Lesions yielding ALH or lobular carcinoma in situ (LCIS) at core needle biopsy with imaging-histologic discordance warrant surgical excision because they have a high rate of cancer in these lesions (38%; 95% CI 9%–76%) on excision. Core needle biopsy with imaging-histologic concordance of ALH or LCIS, the cancer rate was significantly lower (3%; 95% CI 0%–9%); therefore, these do not always mandate surgical excision unless imaging is discordant with pathologic findings.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree