(1)
Global Development, Amgen, Thousand Oaks, CA, USA
(2)
Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Chapter Overview
Owing to improvements in screening and adjuvant therapy, survival following the diagnosis of breast cancer has improved markedly over the past three decades. This chapter will focus on MD Anderson’s recommendations for surveillance and treatment in breast cancer survivors. Because randomized trials have not demonstrated a survival benefit with intensive monitoring, current guidelines support the use of medical history review, physical examination, and annual mammograms as the bedrock of breast cancer surveillance. In addition, given the multidisciplinary approach to breast cancer treatment and surveillance, it is essential to monitor for and treat long-term effects of breast cancer treatment, including lymphedema, cardiac toxicity, ovarian failure, bone disorders, and secondary malignancies.
Introduction
Owing to improvements in screening and adjuvant therapy, survival following the diagnosis of breast cancer has improved markedly over the past three decades (Berry et al. 2005). As a result, an increasing number of breast cancer survivors are requiring evaluation and treatment after the diagnosis of breast cancer. This chapter will focus on MD Anderson’s recommendations for surveillance and treatment in breast cancer survivors.
Surveillance
Type of Monitoring
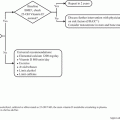
A great concern for breast cancer survivors is the need for close monitoring for recurrent or metastatic disease. However, two large Italian trials, involving an aggregate of more than 2,500 patients with breast cancer, found no improvement in overall survival in patients who underwent intensive surveillance, including physical examination, mammogram, and rigorous tests such as bone scans and chest x-rays, compared with patients who received routine physical examinations and mammograms only (GIVIO Investigators 1994). As a result, current National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and MD Anderson guidelines support surveillance of breast cancer survivors with physical examinations and mammograms; the use of more intensive monitoring is not recommended (see survivorship algorithms for invasive and noninvasive breast cancer, presented at the end of this chapter).
Interval for Monitoring
ASCO recommends that patients undergo a medical history review and physical examination every 3–6 months for the first 3 years following completion of primary therapy; this interval increases to 6–12 months at years 4 and 5 (Khatcheressian et al. 2006). After year 5, patients should undergo the medical history review and physical examination annually, unless earlier evaluation is clinically warranted. NCCN guidelines recommend similar intervals. The surveillance interval pattern used at MD Anderson is similar to that of the ASCO and NCCN guidelines; patients undergo a medical history review and physical examination every 3–6 months for 3 years, every 6–12 months for the next 2 years, and then annually after year 5.
History and Physical Examination
The medical history review and physical examination serve as the primary mechanism for detection of breast cancer recurrence (Lu et al. 2011). The medical history review should include questions that facilitate the detection of local recurrence or metastatic disease, covering the following:
Lumps, nodules, fullness, or skin changes (to detect local recurrence)
Persistent or worsening bone pain (to detect bone metastases)
Abdominal pain, increased abdominal girth, anorexia, or jaundice (to detect liver metastases)
Persistent cough, pleuritic chest pain, or shortness of breath (to detect pulmonary metastases)
New onset or worsening headache, visual changes, nausea, vomiting, dizziness, weakness, bowel or bladder incontinence, or changes in sensation (to detect metastases in the brain or spinal cord)
Changes in bowel habits or alteration in consistency or color of the stool (to detect gastrointestinal metastases)
Pelvic pain or discomfort or new-onset vaginal bleeding or spotting (to detect genitourinary metastases)
Physical examination should involve a complete examination of the patient from head to toe, including a neurologic examination, cardiac examination, pulmonary examination, abdominal evaluation, and evaluation of the breasts and lymph node basins.
Breast Imaging
Mammography remains the primary imaging technique for breast cancer, because it is the only imaging method that has consistently been found to reduce breast cancer–related mortality (Tabar et al. 2001). MD Anderson recommends obtaining a mammogram of a breast treated with breast-conserving therapy after 6 months, and then obtaining a bilateral mammogram annually. For patients who have undergone mastectomy, a mammogram of the contralateral breast should be obtained annually. For patients who have undergone mastectomy and reconstruction, a mammogram is not obtained for the reconstructed breast because mammography of the reconstructed breast has not been shown to increase detection of local recurrence (Fajardo et al. 1993).
Ultrasound is not currently recommended as a primary imaging technique for breast cancer. Instead, it is primarily used as an adjunct to mammography to further evaluate architectural distortion detected by the mammogram, distinguish between a solid mass and a cyst, and assist in localization of a mass or nodule to facilitate biopsy.
The use of magnetic resonance imaging (MRI) of the breast is also increasing. MRI has been found to have greater sensitivity for detection of breast malignancies than mammography, but no current evidence indicates that use of breast MRI improves outcomes when used as a breast surveillance technique (Kuhl et al. 2005). Thus, breast MRI is not routinely recommended for breast cancer surveillance, although it may be used as an adjunct to mammography in patients who have unique characteristics, such as BRCA1/2 mutation carrier status.
Screening for Second Primary Breast Cancers
Breast cancer survivors have a markedly higher risk of developing a second primary breast cancer, compared with the risk of developing primary breast cancer in the general population (Chaudary et al. 1984). Techniques for monitoring for a second primary breast cancer include mammography, ultrasonography, and MRI, as previously described.
Late Effects of Treatment
Surgery and Lymphedema
Mastectomy and axillary lymph node dissection increase the risk of developing lymphedema, which is associated with limb discomfort and decreased quality of life (Beaulac et al. 2002). Furthermore, chronic massive lymphedema may lead to Stewart-Treves syndrome, a rare disease that is associated with the development of lymphangiosarcoma of the involved extremity (Cozen et al. 1999). More commonly, lymphedema of the arm increases the likelihood of skin infections, such as cellulitis, for which close monitoring should be performed.
Chemotherapy
Cardiac Toxicity
Compared with first-generation regimens such as CMF (cyclophosphamide, methotrexate, and 5-fluorouracil), treatment with anthracyclines has been associated with a significant reduction in breast cancer–related mortality and overall mortality (Early Breast Cancer Trialists’ Collaborative Group 2012). However, anthracycline use increases the risk of congestive heart failure in a dose-dependent fashion (Bristow et al. 1981). The risk of anthracycline-related cardiomyopathy increases with age, combination with trastuzumab, and combination with mediastinal radiation therapy (Pinder et al. 2007).
In contrast with anthracycline-related cardiomyopathy, trastuzumab-related cardiotoxicity is often reversible with treatment discontinuation and is not dose-dependent (Keefe 2002). Most often, trastuzumab-related cardiomyopathy is detected by echocardiogram or multigated acquisition scan and is not clinically apparent at the time of diagnosis. Monitoring for cardiac complications from each regimen requires a multidisciplinary approach, with input from each patient’s primary care physician, oncologist, and cardiologist.
Neurologic Toxicity
Review of cross-sectional cognitive outcome studies reveals that the prevalence of chemotherapy-associated cognitive decline ranges from 17% to 75% (Correa and Ahles 2008). Prospective studies of breast cancer survivors undergoing chemotherapy have generated conflicting results, with some studies noting a significant decline in cognitive function and others finding no difference compared with baseline (Wefel et al. 2004b; Shilling et al. 2005; Bender et al. 2006; Hurria et al. 2006; Jenkins et al. 2006; Stewart et al. 2008; Quesnel et al. 2009). However, the patient’s self-perceived cognitive dysfunction is integrally linked to increased psychological distress (Wefel et al. 2004a). Boykoff et al. (2009) published compelling qualitative evidence of the negative effects of “chemobrain” on the economic, emotional, and interpersonal aspects of breast cancer survivors’ lives. Furthermore, a recent prospective study of 101 patients with breast cancer noted that self-perceived cognitive dysfunction was significantly related to negative affectivity (p = .015) and depression (p < .001; Hermelink et al. 2010). Thus, even in the setting of cancer “cure” following chemotherapy, breast cancer survivors continue to face the critical barrier of worsened cognition and its downstream emotional distress in their daily lives.
Ovarian Failure
The risk of chemotherapy-related ovarian failure is related to the dose and type of chemotherapy and the age at diagnosis (Goodwin et al. 1999). Specifically, risk of ovarian failure is markedly increased when the chemotherapy regimen includes cyclophosphamide or anthracycline and is administered to women older than 35 years. Patients with breast cancer may experience hot flashes, vaginal dryness, and mood changes. Early evaluation and symptomatic treatment is essential to facilitate improved quality of life. Furthermore, early ovarian failure increases the risk of osteopenia or osteoporosis, for which close monitoring should occur. Treatment with calcium, vitamin D, and bisphosphonates may be necessary to maintain adequate bone health in this setting (Hillner et al. 2003; see algorithm for breast cancer survivorship bone health, presented at the end of this chapter).
Second Malignancies
Research from our institution has demonstrated a small increased risk of acute myeloid leukemia after adjuvant chemotherapy (1.8% vs 1.2%) in women older than 65 years (Patt et al. 2007). Use of more intense regimens that included two or more cycles containing 2,400 mg/m2 cyclophosphamide with granulocyte colony-stimulating factor support resulted in a cumulative incidence of acute myeloid leukemia of 1.01% (95% confidence interval, 0.63–1.62%), compared with 0.21% (95% confidence interval, 0.11–0.41%) for patients treated with standard AC (doxorubicin and cyclophosphamide) regimens (Smith et al. 2003). Although the benefit from adjuvant chemotherapy exceeds the risk of developing acute myeloid leukemia, appropriate understanding of this risk is necessary for long-term follow-up.
Radiation Therapy
Cardiovascular Toxicity
Historically, postmastectomy radiation was found to increase the risk of cardiovascular toxicity. A large retrospective study of breast cancer survivors demonstrated a significant increase in overall mortality rates in patients who had received postmastectomy radiation; this effect was attributed to deaths from cardiovascular disease (Jones and Ribeiro 1989). However, with advances in radiation therapy and development of adaptive techniques to reduce cardiac exposure to radiation, recent randomized trials evaluating patients who received postmastectomy radiation therapy have shown no increase in cardiovascular morbidity (Hojris et al. 1999). But even with modern radiation therapy techniques, careful monitoring for symptoms of cardiac toxicity remains essential during follow-up visits.
Second Malignancies
Although they are rare, secondary malignancies are a potential late effect of radiation for the treatment of breast cancer. A retrospective study of the Surveillance, Epidemiology, and End Results (SEER) Cancer Incidence Database demonstrated that, at 15 years after diagnosis of breast cancer, the cumulative incidence of angiosarcoma was 0.9 per 1,000 patients who had received radiation therapy, compared with 0.1 per 1,000 patients who had not received radiation therapy (Yap et al. 2002). In patients who have received radiation therapy, an angiosarcoma presents in the irradiated field as a purple macule or papule; clinical suspicion of malignancy should lead to immediate core biopsy for further assessment. In addition to risk of solid tumor malignancies such as angiosarcoma, risk of hematologic malignancies such as acute myeloid leukemia and myelodysplastic syndrome is also slightly increased following radiation therapy (Kaplan et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree