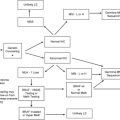
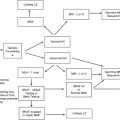
A small, but important, percentage of breast cancer cases is caused by the inheritance of a single copy of a mutated gene. BRCA1 and BRCA2 are the genes most commonly associated with inherited breast cancer; however, mutations in TP53 and PTEN cause Li-Fraumeni syndrome and Cowden syndrome, respectively, both of which are associated with high lifetime risks of breast cancer. Advances in the field of breast cancer genetics have led to an improved understanding of detection and prevention strategies. More recently, strategies to target the underlying genetic defects in BRCA1- and BRCA2-associated breast and ovarian cancers are emerging and may have implications for certain types of sporadic breast cancer.
Increasing numbers of genes have been implicated in the development of breast cancer. Some of these genes are highly penetrant, meaning that a significant proportion of individuals who carry mutations in these genes will develop cancer. Although only 5% to 10% of breast cancers are believed to be caused by mutations in single genes, such mutations predispose carriers to a very high lifetime risk of developing breast cancer. Additionally, women who carry such mutations are at disproportionate risk of developing breast cancer at a young age, before mammographic screening is recommended for the general population.
This review article will focus on the autosomal-dominant, highly penetrant genes TP53 , PTEN , BRCA1 , and BRCA2 . Mutations in these genes are responsible for the hereditary breast cancer syndromes known as Li Fraumeni syndrome (LFS), Cowden syndrome (CS), and hereditary breast and ovarian cancer syndrome (HBOC), respectively.
LFS
LFS is a rare cancer predisposition syndrome characterized by various early onset tumors including breast cancers, sarcomas, brain tumors, adrenal cortical tumors, and acute leukemias ( Table 1 ). Germline mutations in the TP53 gene located on chromosome 17 are identified frequently in this syndrome, with 50% to 70% of families meeting the criteria for classic LFS having detectable mutations. Many more families do not meet the strict criteria for LFS, but meet looser criteria for Li-Fraumeni-like (LFL) syndrome ( Table 2 ). Depending on the criteria met, between 7% to 22% of LFL families have detectable TP53 mutations.
| Li-Fraumeni Syndrome | Cowden Syndrome | Hereditary Breast and Ovarian Cancer Syndrome | ||
|---|---|---|---|---|
| Mutated Gene | TP53 | PTEN | BRCA1 | BRCA2 |
| Gene Location | 17p13.1 | 10q23.3 | 17q21 | 13q12–13 |
| Gene Frequency | As high as 1 in 20,000 | Between 1/200,000 and 1/250,000 | Varies among ethnic groups: 1 in 40 among Ashkenazi Jews Unselected breast cancer patients <5% | |
| Associated, Nonbreast Cancer | Sarcomas Brain tumors Adrenal cortical tumors Acute leukemias Choroid plexus tumors | Thyroid tumors (nonmedullary thyroid cancer) Endometrial cancer Genitourinary tumors, especially renal cell carcinoma | Ovarian/fallopian tube/primary peritoneal cancers Male breast cancer | |
| Prostate | Prostate Pancreatic Gall bladder Stomach Melanoma | |||
| Lifetime Risk of Developing Breast Cancer | 90% by age 60 | 25%–50% | 40%–80% | 30%–60% |
| Associated Syndromes | N/A | Bannayan-Riley-Ruvalcaba syndrome Proteus syndrome | Biallelic mutations in BRCA2 : Fanconi anemia | |
| Li-Fraumeni Syndrome | Li-Fraumeni-Like Syndrome | ||
|---|---|---|---|
| Birch Criteria | Eeles Criteria | ||
| Diagnostic Criteria | Proband with a sarcoma diagnosed before age 45 and A first-degree relative with any cancer <45 and A first- or second-degree relative with any cancer <45, or a sarcoma at any age | Proband with childhood cancer or sarcoma, brain tumor, leukemia or ACC <45 and: First- or second-degree relative with typical Li Fraumeni syndrome cancer and First- or second-degree relative cancer <60 | Two different cancers that are part of Li Fraumeni syndrome in first- or second- degree relative at any age |
| Likelihood of Detecting a TP53 Mutation | 80% | 7%–22%. | |
Inheritance and Penetrance
LFS is inherited in an autosomal-dominant pattern. Although most patients with documented TP53 mutations have an extensive family history of cancer, including childhood cancer, de novo mutations are not infrequent. Approximately 7% to 20% of TP53 mutations are thought to occur de novo. Gonzalez and colleagues estimated that the frequency of TP53 mutations is 1 in 20,000 people, a frequency higher than previously estimated, although still very small. Founder mutations may exist in certain populations, most notably the R337H mutation in Brazil, which may be seen in up to 0.3% of the population. Germline mutations in the TP53 gene are thought to account for only a small fraction of hereditary breast cancers cases and for less than 1% of all breast cancer cases.
LFS is associated with an extremely high lifetime risk of cancer. Women with documented TP53 mutations are more likely to develop cancer than men, with a lifetime risk estimates of 93% and 68%, respectively. The most common cancer in women is early onset female breast cancer. Unlike cases of sporadic breast cancer, which most commonly occur in postmenopausal women, the vast majority of patients with germline TP53 mutations who develop breast cancer do so by age 50, with a mean age of 37. A woman with LFS is thought to have a breast cancer risk of 90%.
Who Should be Tested?
The details of an individual’s personal and family history of cancer are very important in determining the likelihood that a germline TP53 mutation will be detected. An important study by Gonzalez and colleagues examined 525 patients sent for TP53 testing at the City of Hope clinical genetics laboratory (Philadelphia, PA, USA), among whom 91 mutations were detected. In this series, an individual diagnosed with invasive breast cancer between the ages of 30 and 49 years who had no family history of any of the core Li Fraumeni cancers (except breast cancer) had a 0% (0 out of 15) chance of a detectable TP53 mutation. In contrast, a patient with breast cancer under age 30 and family history of one or more core Li Fraumeni cancers (other than breast cancer) in a first- or second-degree relative, had a 100% (five out of five) chance of having a p53 mutation. Probands with adrenal cortical tumors in childhood or choroid plexus tumors had a very high cancer of carrying a TP53 mutation, with prior probabilities of 67% (14 of 21) and 100% (eight of eight), respectively. Women with breast cancer younger than age 30 without any family history of cancer still had a 7% chance of carrying a TP53 mutation, a figure consistent with other series. The limitations of this study include small sample size in the individual subgroups and referral bias; however, the data are helpful in the clinic when considering who should undergo genetic testing for this rare syndrome. Because of the very low frequency of germline TP53 mutations in the general population, women diagnosed with breast cancer after age 30 without a family history suggestive of LFS or Li-Fraumeni-like syndrome (representing the vast majority of breast cancer patients) are very unlikely to have a TP53 mutation.
Screening, Prevention, and Treatment
Postpubescent females with a family history of LFS should be closely monitored for breast cancer. Although there is a theoretical risk of inducing malignancies in TP53 mutation carriers through the exposure to radiation, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology recommend annual mammograms and magnetic resonance imaging (MRI) scans starting at age 20 to 25 for LFS patients, as the benefits of early detection are believed to be greater than their risks ( Table 3 ). Given the high lifetime risk of cancer, prophylactic mastectomy is a reasonable option in TP53 mutation carriers.
| Li-Fraumeni Syndrome | Cowden Syndrome | Hereditary Breast and Ovarian Cancer Syndrome | |
|---|---|---|---|
| Breast Self-examination | Monthly, starting at age 18 | ||
| Clinical Breast Examination | Semiannually, starting at age 20–25 or 5–10 years before the earliest known breast cancer in the family | Semiannually, starting at age 25 or 5–10 years before the earliest known breast cancer in the family | Semiannually, starting at age 25 |
| Breast Radiographic Screening | Annual mammogram and breast magnetic resonance imaging (MRI) screening starting at age 20–25 or individualized based on earliest age of onset in family | Annual mammography and breast MRI screening starting at age 30–35 or 5–10 years before the earliest known breast cancer in the family (whichever is earlier) | Annual mammogram and breast MRI screening starting at age 25 or individualized based on earliest age of onset in family |
| Prophylactic Surgery | Discuss option of risk-reducing mastectomy (RRM) | Discuss option of RRM | Discuss option of RRM Recommend RRSO, ideally between 35 and 40 years of age |
| Screening for Other Cancers | Colonoscopy every 2–5 years starting at 25 Annual comprehensive examination and neurologic examinations Ongoing investigations regarding the role of positron emission tomography or MRI | Baseline thyroid ultrasound, consider yearly thyroid ultrasound Role of yearly endometrial biopsy is debated: not included in current NCCN guidelines | Twice yearly TVUS and CA125 for ovarian cancer screening until the time RRSO Consider yearly dermatology examination in BRCA2 mutation carriers Discussion of prostate cancer screening in male carriers |
Screening Guidelines for Other Cancers
Individuals with germline TP53 mutations are at elevated risks for many types of cancer. For this reason, there is great interest in developing effective screening strategies. Garber and colleagues assessed the utility of positron emission tomography/computed tomography (PET/CT) in a small cohort of TP53 mutation carriers. Cancers were detected in 3 of 15 patients undergoing PET/CT, including two papillary thyroid cancers and a stage 2 esophageal adenocarcinoma. There is significant interest in the potential role of full-body MRI. Individuals with this rare syndrome are encouraged to enroll on clinical studies. Annual comprehensive examinations should be performed with a focus on skin and neurologic examinations. Colonoscopy should be considered starting at age 25 (see Table 3 ).
Therapeutic Implications
Patients who carry a germline TP53 mutation and are diagnosed with breast cancer should consider mastectomy rather than breast-conserving surgery and radiation. Increased sensitivity to DNA-damaging agents such as ionizing radiation may place individuals with TP53 mutations at higher risk for developing treatment-induced cancers, particularly sarcomas in the radiation field.
CS
CS, also known as multiple hamartoma syndrome, is a rare cancer predisposition associated with a high rate of breast cancer and mucocutaneous findings, thyroid abnormalities, and endometrial carcinomas (see Table 1 and Table 4 ). The diagnostic criteria for CS are complex, but are summarized in Table 4 . Germline mutations in the PTEN gene have been found to occur in 80% of strictly defined CS patients. The PTEN gene encodes the PTEN protein, a lipid phosphatase enzyme involved in the regulation of the cell cycle and apoptosis. PTEN , like TP53 , functions as a tumor suppressor gene.
| Definition | Any single pathognomonic criterion or ≥2 major criteria or 1 major and ≥3 minor criteria or ≥4 minor criteria |
| Criteria | |
| Pathognomonic | Mucocutaneous lesions Facial trichilemnomas Acral keratosis Papillomatous lesions Mucosal lesions Adult Lhermitte Duclos disease |
| Major | Breast cancer Endometrial cancer Thyroid cancer Macrocephaly |
| Minor | Fibrocystic breast disease Mental retardation Benign thyroid lesions Hamartomatous polyps Lipomas Fibromas GU tumors Fibroids Renal cell carcinoma |
Inheritance and Penetrance
CS is an autosomal-dominant, highly penetrant genetic disorder. More than 90% of individuals with PTEN mutations are believed to manifest some feature of the syndrome (although rarely cancer) by age 20, and by age 30 nearly 100% of carriers are believed to have developed at least some of the mucocutaneous signs.
Women with CS have approximately a 67% to 76% risk for benign breast disease, such as fibroadenomas and fibrocystic breast disease, and a 25% to 50% lifetime risk for breast cancer. The peak incidence of breast cancer in women with CS occurs between the ages of 38 and 46 years, although there is a wide range in age of onset. Like LFS, the exact prevalence of CS is unknown, although its prevalence has been estimated to be between 1 case in 200,000 people and 1 case in 250,000 people based on projections from a study of the Dutch national registry. CS, though undoubtedly rare (it is estimated that fewer than 1% of hereditary breast cancer families are caused by CS), may be under-recognized, as women who present with breast cancer and subtle skin findings or common associated features (ie, fibroids or fibrocystic breast disease) may not be recognized as potentially part of the Cowden’s spectrum.
Familial data collected by Nelen and colleagues show that there is marked variation in symptom manifestation and age of symptom occurrence in patients with CS. In part because of this phenotypic heterogeneity, and in part because of the occurrence of de novo PTEN germline mutations, a family history of breast cancer may not be apparent. For example, in one study of 19 women with breast cancer and CS, only 35% of the women reported a family history of breast cancer.
Screening, Prevention, and Treatment
Women with CS should be monitored closely for breast cancer (see Table 3 ). Breast cancers seen in women with germline PTEN mutations show a spectrum of pathology similar to that of sporadic breast cancers in the general population, with the predominant type of breast cancer being ductal carcinoma. Annual screening breast MRI in addition to annual mammograms are recommended for women with PTEN mutations starting in their early 30s. Similar to those with TP53 mutations, screening and prevention information specific to those with PTEN mutations are limited because of the rarity of the syndrome. Individuals with PTEN mutations are at elevated risk for follicular and papillary carcinoma of the thyroid, and a baseline screening thyroid ultrasound at 18 with consideration of annual ultrasound has been suggested. In addition, because of the increased risk of endometrial cancer (with a potential lifetime risk of 5% to 10%), yearly endometrial biopsy starting at age 35 to 40 years has been recommended by some authors but is not currently part of the NCCN guidelines. The increased risk of developing endometrial cancer with tamoxifen use is of particular concern to those with PTEN mutations as such women are already at an increased risk for endometrial cancer. Therefore, if women are interested in primary prevention, raloxifene, with no apparent excess risk of uterine cancer, may be preferred over tamoxifen. As is true for any women at increased risk for the development of breast cancer, prophylactic mastectomy is an option for women with PTEN mutations.
CS
CS, also known as multiple hamartoma syndrome, is a rare cancer predisposition associated with a high rate of breast cancer and mucocutaneous findings, thyroid abnormalities, and endometrial carcinomas (see Table 1 and Table 4 ). The diagnostic criteria for CS are complex, but are summarized in Table 4 . Germline mutations in the PTEN gene have been found to occur in 80% of strictly defined CS patients. The PTEN gene encodes the PTEN protein, a lipid phosphatase enzyme involved in the regulation of the cell cycle and apoptosis. PTEN , like TP53 , functions as a tumor suppressor gene.
| Definition | Any single pathognomonic criterion or ≥2 major criteria or 1 major and ≥3 minor criteria or ≥4 minor criteria |
| Criteria | |
| Pathognomonic | Mucocutaneous lesions Facial trichilemnomas Acral keratosis Papillomatous lesions Mucosal lesions Adult Lhermitte Duclos disease |
| Major | Breast cancer Endometrial cancer Thyroid cancer Macrocephaly |
| Minor | Fibrocystic breast disease Mental retardation Benign thyroid lesions Hamartomatous polyps Lipomas Fibromas GU tumors Fibroids Renal cell carcinoma |
Inheritance and Penetrance
CS is an autosomal-dominant, highly penetrant genetic disorder. More than 90% of individuals with PTEN mutations are believed to manifest some feature of the syndrome (although rarely cancer) by age 20, and by age 30 nearly 100% of carriers are believed to have developed at least some of the mucocutaneous signs.
Women with CS have approximately a 67% to 76% risk for benign breast disease, such as fibroadenomas and fibrocystic breast disease, and a 25% to 50% lifetime risk for breast cancer. The peak incidence of breast cancer in women with CS occurs between the ages of 38 and 46 years, although there is a wide range in age of onset. Like LFS, the exact prevalence of CS is unknown, although its prevalence has been estimated to be between 1 case in 200,000 people and 1 case in 250,000 people based on projections from a study of the Dutch national registry. CS, though undoubtedly rare (it is estimated that fewer than 1% of hereditary breast cancer families are caused by CS), may be under-recognized, as women who present with breast cancer and subtle skin findings or common associated features (ie, fibroids or fibrocystic breast disease) may not be recognized as potentially part of the Cowden’s spectrum.
Familial data collected by Nelen and colleagues show that there is marked variation in symptom manifestation and age of symptom occurrence in patients with CS. In part because of this phenotypic heterogeneity, and in part because of the occurrence of de novo PTEN germline mutations, a family history of breast cancer may not be apparent. For example, in one study of 19 women with breast cancer and CS, only 35% of the women reported a family history of breast cancer.
Screening, Prevention, and Treatment
Women with CS should be monitored closely for breast cancer (see Table 3 ). Breast cancers seen in women with germline PTEN mutations show a spectrum of pathology similar to that of sporadic breast cancers in the general population, with the predominant type of breast cancer being ductal carcinoma. Annual screening breast MRI in addition to annual mammograms are recommended for women with PTEN mutations starting in their early 30s. Similar to those with TP53 mutations, screening and prevention information specific to those with PTEN mutations are limited because of the rarity of the syndrome. Individuals with PTEN mutations are at elevated risk for follicular and papillary carcinoma of the thyroid, and a baseline screening thyroid ultrasound at 18 with consideration of annual ultrasound has been suggested. In addition, because of the increased risk of endometrial cancer (with a potential lifetime risk of 5% to 10%), yearly endometrial biopsy starting at age 35 to 40 years has been recommended by some authors but is not currently part of the NCCN guidelines. The increased risk of developing endometrial cancer with tamoxifen use is of particular concern to those with PTEN mutations as such women are already at an increased risk for endometrial cancer. Therefore, if women are interested in primary prevention, raloxifene, with no apparent excess risk of uterine cancer, may be preferred over tamoxifen. As is true for any women at increased risk for the development of breast cancer, prophylactic mastectomy is an option for women with PTEN mutations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree