Fig. 6.1
Current incidence and projected number of new cancer in sub-Saharan Africa by 2035 (Ferlay et al. 2013)
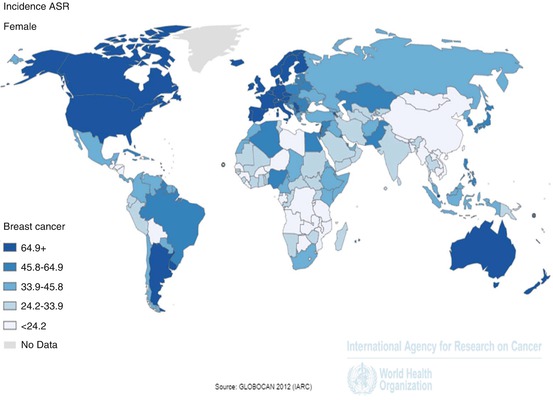
By international standards, breast cancer in SSA is not common (Chokunonga et al. 2013; Rambau et al. 2011), with an incidence approximately one quarter that of the US (Fig. 6.2), (Huo et al. 2009). This difference may be at least partly racially-driven: it has been shown that white South Africans have a rate of breast cancer more than six times that of their black counterparts (Vorobiof et al. 2001), and similar findings have been reported in the US (Stark et al. 2010; Fregene and Newman 2005; Adebamowo et al. 2008). In both societies, though, the effects of socioeconomic inequality are difficult to disentangle.
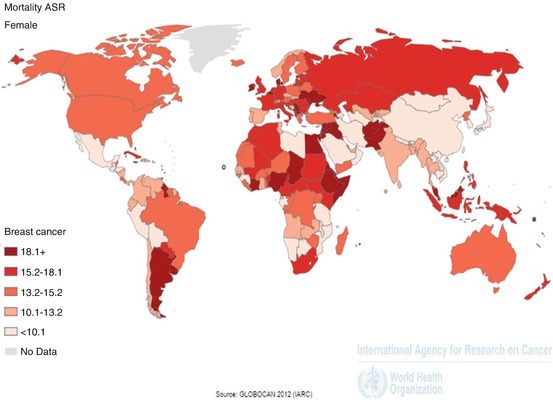
SSA breast cancer may be more aggressive than in the West. It is notable that, despite dramatic differences in incidence, the mortality rates from breast cancer are very similar in the US and SSA (Fig. 6.3), (Huo et al. 2009). Moreover, higher mortality rates have been reported in US African-Americans, compared to Caucasians (Stark et al. 2010; Fregene and Newman 2005). The aggressive inflammatory breast cancer has also been reported to be more common in African women (McCormack et al. 2013).
The mean age of SSA women with breast cancer has consistently been shown to be approximately 10–15 years lower than their European and Northern American counterparts (Rambau et al. 2011), with the disease affecting predominantly premenopausal women in much of the region (Sighoko et al. 2013; Chokunonga et al. 2013; Titloye et al. 2016). Evidence that this is racially-driven derives from studies of African-American women, who also develop breast cancer at a younger age than Caucasians (Fregene and Newman 2005; Stark et al. 2010). However, many investigators have suggested that this phenomenon is simply a reflection of the fact that few women in SSA live beyond their sixth decade, artificially reducing the mean age of women afflicted by breast cancer (Gukas et al. 2005; Akarolo-Anthony et al. 2010). Interestingly, with rapid increases in life expectancy across SSA, it has been noted that most of the rising incidence of the disease is being driven by increased rates in older women (Parkin et al. 2009; Chokunonga et al. 2013; Akarolo-Anthony et al. 2010); if these trends continue, it is plausible that the demographics of breast cancer in SSA will come to resemble those of Western societies.
Although data are scarce, it is worth recognising that rates of male breast cancer in SSA have consistently been shown to be significantly higher than in Western societies. Studies in Ghana, Nigeria, Tanzania and Uganda have identified rates of 2.4–8.6% (Akosa et al. 2005; Gakwaya et al. 2008; Kidmas et al. 2005), compared to less than 1% in Caucasian populations (Speirs and Shaaban 2009).
6.3 Risk Factors
It is widely-accepted that breast cancer risk is correlated with lifetime exposure to oestrogen; thus, the traditional reproductive patterns of women in SSA – late menarche, multiple pregnancies from an early age, and exclusive breastfeeding for more than a year – may have been responsible for the historically low incidence of the disease (Okobia et al. 2006b). Many investigators have demonstrated that breast cancer in SSA is associated with delayed first pregnancy, reduced numbers of pregnancies, and reduced duration of breastfeeding (Akarolo-Anthony et al. 2010; Parkin et al. 2009; Chokunonga et al. 2013; Huo et al. 2008; Okobia et al. 2006b; Adebamowo et al. 2002). Interestingly, some studies have shown that early first pregnancy is paradoxically associated with increased cancer risk in pre-menopausal SSA women. This has been explained by the fact that there is a known, transient increase in breast cancer risk following pregnancy (Sighoko et al. 2013; Jordan et al. 2013); historically, interparous intervals in SSA have been short and women have not survived long after menopause, meaning that they suffer from the increased post-parous cancer risk without ever benefiting from the long-term protective effect – this may contribute to the low mean age of breast cancer in SSA (Huo et al. 2008; Okobia et al. 2006b). It is to be noted that the protective effect of pregnancy and breast feeding relates to oestrogen receptor positive breast cancer and has no effect on reducing the likelihood of hormone receptor negative breast cancer (Phipps et al. 2011).
Given rapid improvements in nutrition in the region, anthropometric risk factors have been examined with increasing interest. Contradictory findings have been reported in relation to the relationship between breast cancer and obesity in SSA (Chokunonga et al. 2013; Adebamowo et al. 2002). A possible reason for this is that patients often present with advanced disease, commonly associated with cachexia, rendering cause and effect difficult to disentangle (Brinton et al. 2014; Okobia et al. 2006b). Other researchers have found that high hip-to-waist ratios are more strongly correlated with an increased risk of breast cancer (Okobia et al. 2006b) and, importantly, that this effect is seen both in early- and late-stage cancers (Adebamowo et al. 2002).
Interestingly, it has been proposed that a high BMI in early life is associated with a subsequent increased breast cancer risk (Jordan et al. 2013) and several studies have identified a positive relationship between height and breast cancer risk (Adebamowo et al. 2002). Adult height is strongly related to nutritional status in childhood which is, itself, related to age of menarche. As nutrition improves in SSA, the age of menarche has fallen and this has been associated with increased cancer risk (Akarolo-Anthony et al. 2010; Parkin et al. 2009; Huo et al. 2008; Adebamowo et al. 2008).
6.4 Clinical and Pathological Features
Almost without exception, studies have shown that the breast cancers of SSA women are larger and more advanced than those in their Western counterparts. Mean tumour size has been reported to be 3–7 cm (Brinton et al. 2014; Rambau et al. 2011; Stark et al. 2010; Titloye et al. 2016), and 53–91% of patients have presented at stage III or IV (Brinton et al. 2014; Kantelhardt et al. 2014; Rambau et al. 2011). Investigators have reported 70–92% of patients having lymph node metastases at presentation (Ikpatt et al. 2002a; Rambau et al. 2011; Brinton et al. 2014), 39–46% of women having fungating cancers (Clegg-Lamptey and Hodasi 2007; Adesunkanmi et al. 2006) and 13% having distant metastases (Adesunkanmi et al. 2006).
Of course, that this truly reflects a greater degree of biological aggressiveness cannot necessarily be assumed; relatively poor access to quality diagnostics and treatment undoubtedly allows tumours to progress by the time of presentation. Two early findings, however, lend some weight to the suggestion that SSA breast cancer is indeed intrinsically more aggressive: that 81% of women even with a less than 3 month symptom history had stage III or IV disease at presentation (Hassan et al. 1992), and that 77.7% black South African women presented with stage III or IV disease compared to 30.7% of their white counterparts (Vorobiof et al. 2001).
From a pathological perspective, grade 3 cancers constitute the majority of tumours in most series (Brinton et al. 2014), (Fig. 6.4a, b). Studies examining histological types of breast cancer have presented complex, often contradictory results, owing perhaps to relatively small numbers of cases. In all studies, ductal carcinoma of no special type (NST) has been the most common subtype, accounting for up to 92.7% of cases (Gukas et al. 2005). It is difficult to comment on the incidence of more uncommon subtypes, but a fairly consistent feature in the literature is the underrepresentation of lobular carcinoma, making up only 0–5.2% of cases (Ikpatt et al. 2002b; Kantelhardt et al. 2014; Adebamowo et al. 2008; Rambau et al. 2011; Clegg-Lamptey and Hodasi 2007; Titloye et al. 2016); it has been suggested that this reflects the fact that lobular carcinoma is generally a disease of older women who are less represented in SSA cohorts (Gukas et al. 2005). Given the lack of mammographic screening, it is unsurprising that isolated DCIS diagnoses are rare in SSA (Rambau et al. 2011; Bird et al. 2008).
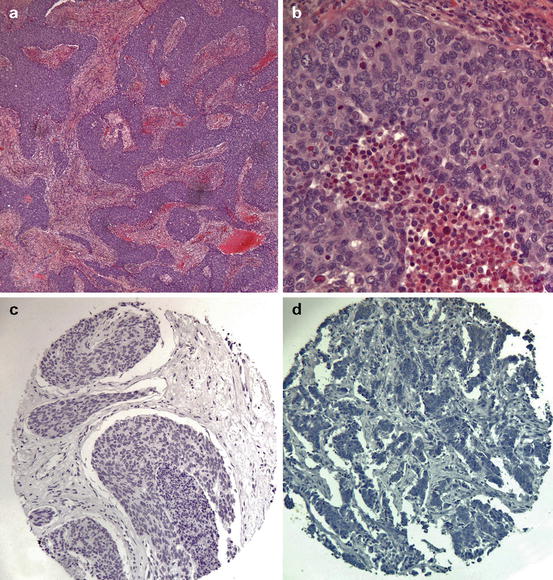

Fig. 6.4
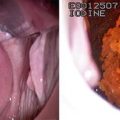
Typical morphological and immunohistochemical features of sub-Sarahran breast cancer. (a) Low-power appearance of a typical high-grade tumour showing a syncytial growth pattern. (b) High-power appearance showing large pleomorphic nuclei with conspicuous mitoses and stromal chronic inflammation. (c) Tissue microarray showing oestrogen receptor (ER) negativity. (d) Tissue microarray showing HER2 negativity
6.5 Molecular Biology
Improved understanding of the molecular biology of breast cancer, and the relationship of molecular markers with biological behaviour, has led to a conception of breast cancer as a constellation of related but distinct diseases. Breast cancer has been classified into five taxonomic subtypes (Perou et al. 2000) which are related to protein expression on immunohistochemistry, based on hormone receptor, HER2, proliferation index and basal marker expression. A summary of the prevalence of these molecular subtypes across various ethnic backgrounds is presented in Table 6.1.
Table 6.1
Summary of molecular profiles of breast cancer in the UK, sub-Saharan Africa and African-Americans in the US
Study | Galukande et al. (2014) | Titloye et al. (2016) | Adebamowo et al. (2008) | Galukande et al. (2014) | Ihemelandu et al. (2007) | ||
Location | UK | Nigeria | Nigeria | Nigeria | Uganda | USA | |
Population | Not stated | Not stated | Black African | Not stated | Not stated | African Americans | |
Age | 32% ≤ 50 years | 61% ≤ 50 years | 85% ≤ 50 years | Not stated | Mean age 45 years | ≤ 65 years old | < 35 years only |
Luminal A | 76 | 26 | 15 | 78 | 38 | 55 | 26 |
Luminal B | 5 | 5 | 5 | 3 | 5 | 12 | 14 |
HER2 | 5 | 19 | 20 | 4 | 22 | 12 | 4 |
Triple-negative | 10 | 38 | 60 | 16 | 34 | 21 | 57 |
The luminal subtype, which is characterised by ER and PR expression, is dominant in Caucasian populations, but is relatively uncommon in SSA (Bird et al. 2008; Stark et al. 2010; Gukas et al. 2005; Ikpatt et al. 2002b). Instead, SSA populations are dominated by triple-negative and HER2+ subgroups. However, it has been noted that techniques used to assess immunohistochemical status in older studies have been suboptimal: many rely on the use of archival material in which antigen degradation is well-recognised, use of large resection specimens rather than biopsy material is frequent, under- and over-fixation are both common, and robust internal quality control is often lacking (Adebamowo et al. 2008; Akarolo-Anthony et al. 2010; Brinton et al. 2014; McCormack et al. 2013). Ironically, recent studies which have adhered meticulously to immunohistochemical protocols have continued to identify variable proportions of the different molecular profiles (Adebamowo et al. 2008; Huo et al. 2009; Titloye et al. 2016).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree