Fig. 8.1
(a) Proportion of all cancer in females that is breast cancer by single year of age, 2000–2012, SEER18. (b) Incidence of breast cancer in females by single year of age, 2000–2012, SEER18
The incidence of breast cancer appears to have a sigmoid function in women less than 55 years of age (Fig. 8.1b), with 6.2 % of all cases diagnosed before age 40, 2.3 % diagnosed before age 35 and <1 % diagnosed before age 30 (Fig. 8.2, inset). The incidence of breast cancer in AYA women is fairly similar between different countries worldwide, with no clear differences in between Westernized and developing countries [5]. During 2012 in the USA, the individual average risk of a woman developing invasive breast cancer was 1 in 166 by the age of 40 and approximately 1 in 419 by the age of 35 (Table 8.1). The corresponding rates for being diagnosed with either invasive or in situ breast cancers were 1 in 142 and 1 in 381 (Table 8.1).
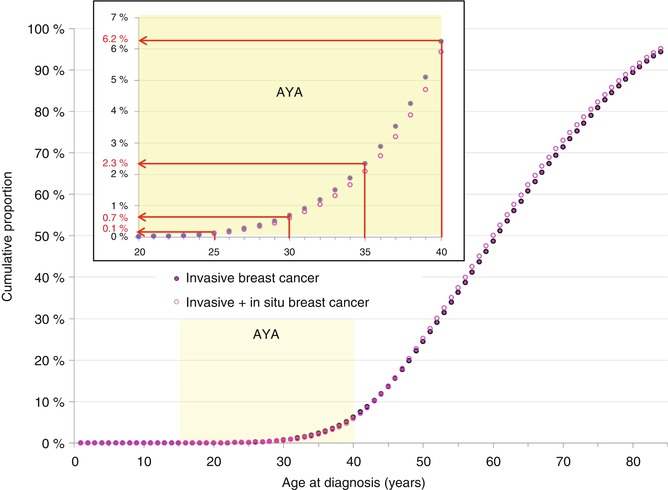

Fig. 8.2
Cumulative proportion of number of women with breast cancer by single year of age, 2000–2012, SEER18
Table 8.1
Risk of invasive and all breast cancer in women during 2012 as a function of age in the USA estimated from 18 SEER regions
Age (years) | I in x | |
|---|---|---|
Invasive | Invasive or in situ | |
15 | 196,078 | 196,078 |
20 | 42,735 | 42,735 |
25 | 7,452 | 6,592 |
30 | 1,392 | 1,284 |
35 | 419 | 381 |
40 | 166 | 142 |
45 | 80 | 63 |
50 | 45 | 34 |
55 | 29 | 23 |
60 | 21 | 16 |
65 | 15 | 12 |
70 | 11 | 9 |
75 | 9 | 7 |
80 | 8 | 6 |
85 | 7 | 5 |
In situ breast cancer increased after screening mammography was initiated nationally in the early 1980s in the USA with a baseline screen recommended at age 35 and the increased use of ductal carcinoma in situ (DCIS) as a pathologic diagnosis (Fig. 8.3). Since then, however, the incidence of all invasive breast cancer has not increased in AYA women since 1981 (Fig. 8.3, purple data). All invasive and DCIS increased slightly at a statistically significant average present change (APC) of 0.34 (Fig. 8.3, brown data). The incidence of localized and regional disease has remained constant since 1976 (Fig. 8.4). The incidence of distant disease in American women <40 years of age, however, has been increasing since 1976–1984 (Fig. 8.3, upper panel) [7]. In a study of 37 European countries, the incidence of breast cancer in women under age 40 increased by an average of 1.2 % percent per year between 1990 and 2008, with the largest increases in women under 35 [6].
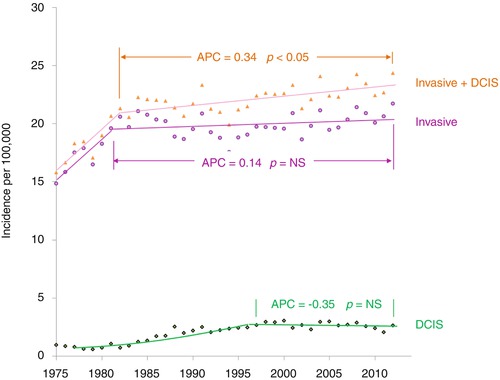
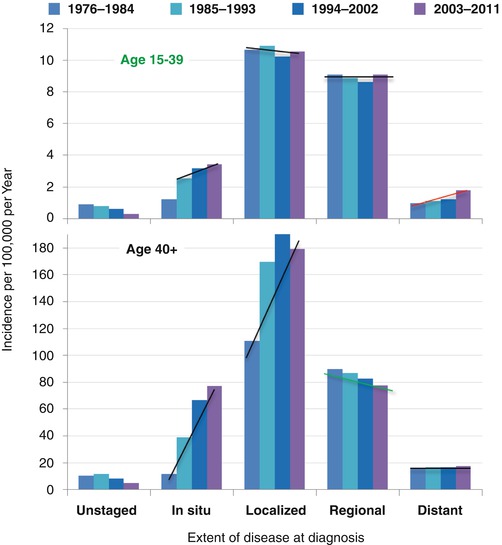

Fig. 8.3
Annual incidence of invasive breast cancer, invasive breast cancer plus DCIS, and DCIS in 15- to 39-year-old women by year of diagnosis, SEER9

Fig. 8.4
Incidence trends in women with breast cancer by extent of disease at diagnosis, 1976–2011, age 15–39 and 40+, SEER9
In American women 40 years of age or older, the incidence of in situ and localized disease increased dramatically since 1976–1984, whereas regional disease has had a moderate decrease and distant disease has had no decrease at all (Fig. 8.3, lower panel). The striking increase in in situ and localized disease in women over 40 years of age is due to screening mammography that is routinely performed in the USA when women reach the age of 40.
SEER data show that the incidence of localized and regional disease in AYA women has remained constant since 1976 (Fig. 8.4). The incidence of distant (stage IV) disease at diagnosis is higher in AYAs than in older women (Fig. 8.4) and has been increasing over the past 30 years in the USA [7]. A smaller percentage of AYA compared to older women are diagnosed with localized (stage 0 or I) disease. Two of every three 25- to 29-year-olds have nonlocalized regional or advanced cancer (stage II, III, or IV) at diagnosis in comparison to slightly more than one in three women 40 or more years of age (Fig. 8.5). In older women, the decline in incidence in 2008–2011 has been attributed to reductions in the use of hormone replacement therapy (Fig. 8.4) [8].
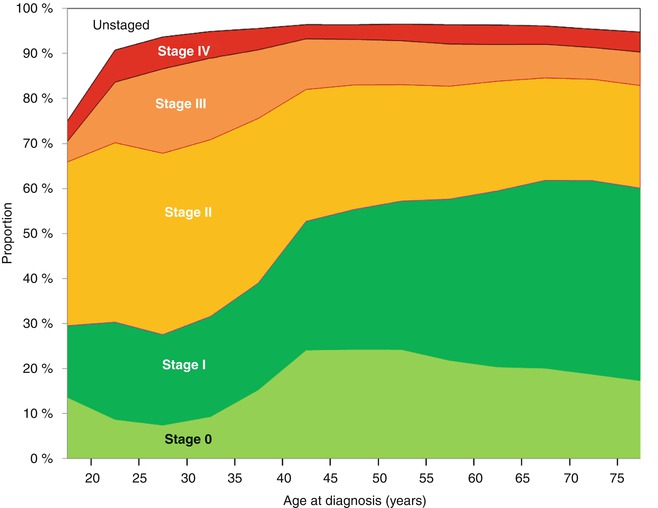

Fig. 8.5
Incidence of breast cancer in females by stage (AJCC6 5 categories), 2004–2011, SEER18
The incidence of breast cancer in young women in the USA also varies according to race and ethnicity. In women over 45, breast cancer is more common in whites than blacks. However, AYA black women under age 35 have a higher incidence of invasive breast cancer and three times the breast cancer mortality of young white women (SEER 18, and National Center for Health Statistics, data not shown [9–11]. In contrast, Native American women at all ages have a lower incidence of breast cancer compared to the general population (RR = 0.7 for women 20–44 years of age) [12]. Women of all age groups with low socioeconomic status as well as young black, Hispanic, and Native American women have an increased likelihood of presenting with advanced disease [13–15].
The overall incidence of breast cancer in males is approximately 1/100th that of the rate in females. Analysis of the SEER 9 database (data not shown) indicates that the incidence of breast cancer has increased for older men but not AYA men during the past three decades. For invasive breast cancer, female AYAs have a worse survival than either older females or AYA males, who have a better survival than older males (Fig. 8.6). One quarter of men with breast cancer die of the disease within 10 years. The age dependence of breast cancer differs significantly between men and women, with an older age predominance seen in men and only 2.6 % of cases occurring before age 40. Breast cancer in males is not further reviewed in this article.
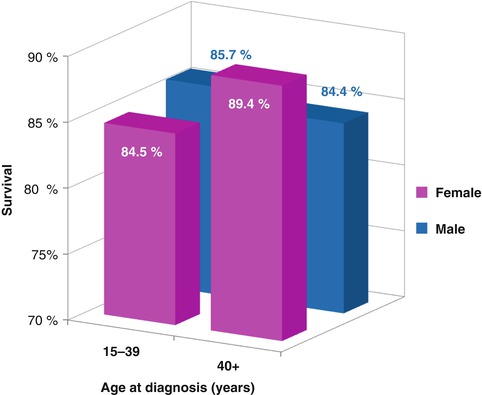

Fig. 8.6
Invasive breast cancer 5-year relative survival by sex and age, 2000–2012 SEER
8.2.2 Risk Factors
8.2.2.1 Familial
A positive family history of cancer is a very strong risk factor for women under 35 (RR, 3.22) and suggests the presence of a familial cancer syndrome [9]. Early-onset breast cancer in AYAs is more likely to be associated with a positive family history of breast cancer, especially in women harboring a germline BRCA1 mutation [16]. Patients with BRCA1 mutations develop aggressive triple-negative tumors in about 80 % of cases. Conversely, constitutional BRCA mutations are found in 11–16 % of women diagnosed with triple-negative breast cancer [17]. In a study of women with breast cancer diagnosed before age 30, BRCA1, BRCA2, and TP53 mutations were found in about half of patients with strong family histories of breast cancer but less than 10 % of women with negative family history of breast cancer [18]. Compared to the general population, women with constitutional PALB2 mutations have at least an eightfold increased risk of developing breast cancer under 40 years of age [19]. Women with constitutional PTEN mutations (Cowden’s syndrome) also have an increased risk for early breast cancer [20].
8.2.2.2 Hormonal
Some hormonal risk factors are similar between AYA and older women. Breast cancer risk is increased by later age (>30 years) at first birth (RR, 1.10; 95 % CI, 0.80–1.50 for women under age 40), early menarche (age <40: RR, 0.93; 95 % CI, 0.87–0.99 for each additional year after age 12 for onset of at menarche), and breastfeeding (age <40: RR, 0.84; 95 % CI, 0.57–1.22) [21].
Other hormonal risk factors are somewhat different for AYA in comparison to older women. For women aged less than 35, recent oral contraceptive use is a risk factor for early-onset breast cancer (RR, 2.26), particularly for ER-negative tumors [22]. For AYAs under 40, current oral contraceptive use for ≥5 years prior to diagnosis is associated with and increased risk of ER(−) tumors (OR, 3.5; 95 % CI, 1.3–9.0) and triple-negative tumors (OR, 3.7; 95 % CI, 1.2–11.8) [23]. BRCA1 carriers who use oral contraceptives prior to age 20 have an increased risk for early-onset breast cancer before age 40 (OR, 1.45; 95 % CI 1.20–1.75; p = 0.0001). Risk increases by 11 % for each additional year of contraceptive use prior to age 20 [24].
Childbearing and multiparity are risk factors, due to a short-term elevation in breast cancer risk for several months immediately following a birth [22]. There is a positive association between high estradiol levels in the first trimester of pregnancy and risk of hormone receptor-negative cancer diagnosed under age 40 . Women with anovulatory infertility are at high risk for early-onset breast cancer [26].
8.2.2.3 Personal and Lifestyle
The combination of obesity, high-energy (caloric) intake, and sedentary lifestyle imparts a higher risk of breast cancer for premenopausal women [27]. Large hip circumference (whether or not abdominal obesity is present) is also a risk factor for premenopausal breast cancer [hazard ratio (HR), 2.85; 95 % CI, 1.33–6.13] for ER−/PR− tumors before and also after adjustment for BMI and for ER + PR+ tumors after adjustment for BMI [HR, 1.65; 95 % CI, 1.04–2.62].
Other risk factors for breast cancer in premenopausal women include a history of prior mantle irradiation for Hodgkin lymphoma, heavy alcohol consumption, and a high intake of red meat [9, 20, 28]. Intense physical activity and a high intake of certain fruits and vegetables (e.g., citrus fruits, cruciferous vegetables [29], tomatoes) are associated with a decreased breast cancer risk in premenopausal women [30–32]. Substituting legumes for one serving of meat per day was associated with a 19 % lower risk of breast cancer in premenopausal women (0.81; 95 % CI, 0.66–0.99) [33].
Increased mammographic density is a risk factor for breast cancer at all ages [34]. Breast density in premenopausal women is mediated by intake of vitamin D, calcium, dietary fat, and alcohol in premenopausal women [35]. Mammographic density is genetically mediated, but no causative genes have been conclusively identified. Genetic variations in IL6 correlate with mammographic density [36]. Also, high levels of endogenous sex hormones such as dehydroepiandrosterone sulfate (DHEAS) in early adolescence have been positively associated with breast density during young adulthood [37].
Several studies suggest that high vitamin D intake, with or without calcium, may protect against premenopausal breast cancer. Low calcium/vitamin D intake has been associated with larger and higher-grade breast tumors [38–40]. Premenopausal women who consumed at least 400 IU vitamin D and 1,000 mg calcium daily were noted to have an 8.5 % (95 % CI, 1.8–15.1) lower mean breast density than those who consumed less [41]. One study noted an inverse association between plasma 25-hydroxyvitamin D level and breast cancer risk in premenopausal women with high mammographic density. No significant association was noted for premenopausal women with lower mammographic density [42].
In a series of women with breast cancer aged 20–44, significant weight gain (BMI increase of >10 kg/m2) after age 18 conferred a 2.0-fold increased risk of ER-negative breast cancer (95 % CI, 1.2-fold to 3.3-fold increased risk). Height, current weight, and weight at 18 years of age had no effect on risk. Smoking history of >10 pack-years increased risk of ER-positive breast cancer by 1.6-fold (95 % CI, 1.1-fold to 2.4-fold increased risk), but did not increase risk of triple-negative breast cancer [43].
8.3 Clinicopathologic Features, Biology, and Prognosis
Clinicopathologic and prognostic features of breast cancer arising in young women compared with their older counterparts have been the subject of published studies for decades [44–46]. Traditionally, breast cancer arising in a younger host aged less than 40 is characterized by a more aggressive phenotype. Among 185 premenopausal women with a diagnosis of invasive breast cancer at the European Institute of Oncology from April 1997 to August 2000, those aged less than 35 had a higher percentage of estrogen receptor (ER)-negative (p < 0.001), progesterone receptor (PR)-negative (p < 0.001), and pathologic grade 3 tumors (p < 0.0001), as well as higher incidence of vascular or lymphatic invasion (48.6 % versus 37.3 %, p = 0.006) compared with women aged 35–50 years [47]. Differences in tumor size, lymph node involvement, and Her2/neu status between younger and older women diagnosed with breast cancer have historically been less clear [47–50].
Younger age has been shown in several studies to be an independent predictor of adverse outcome [48, 49, 51–53]. A retrospective evaluation of over 1,200 women diagnosed with early-stage breast cancer showed young age (<35 years) was an independent prognostic factor for time to recurrence (RR, 1.70, p < 0.001), time to distant failure (RR, 1.60, p < 0.009), and overall mortality (RR, 1.50, p < 0.04) in multivariable analysis [52]. A second retrospective study evaluating over 200,000 women in the SEER database diagnosed with breast cancer between the years of 1988–2003 revealed that those under the age of 40 were 39 % more likely to die when compared to those 40 or older (HR, 1.39; 95 % CI, 1.34–1.45). Moreover, the highest mortality disparity between younger (<40 years) and older women (≥40 years) was present in early-stage, rather than later-stage disease [54, 55]. Although disparities in outcome between younger and older women diagnosed with breast cancer have been attributed traditionally to adverse prognostic features and later stage at diagnosis, this report implicates biologic underpinnings that may unify breast cancer arising in the younger host.
Several comprehensive, large-scale genomic analyses have been conducted to examine the biology of breast cancer arising in young women with the goal of providing insight into this historically aggressive disease process. Azim et al. aimed to define the role of gene expression signatures in the prediction of prognosis in young women and help elucidate biological differences according to age [53]. Using a three-gene classifier (ESR1, ERBB2 [HER2], and AURKA) and defining age ≤40 as young, gene expression data from over 3,500 breast tumors illustrated a higher proportion of young women’s tumor were triple negative (34.3 % vs. 17.9 %). Younger women had a higher risk of relapse compared to the older age group, a comparison that held true after adjusting for tumor size, nodal status, grade, and breast cancer subtype in multivariable analysis (p = 0.04). Moreover, the prognostic value of three proliferation-related, three stromal-related, and three immune signatures were evaluated by age and subtype. The most notable finding was that the stromal-related signatures showed a significant interaction with age in the triple-negative subtype of patients (HR, 2.4; p, 0.04). Finally, using a candidate gene approach and after adjusted for subtype, over 12 gene sets were found to be associated with young age including those related to an immature mammary cell population, growth factors (i.e., mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)), and downregulation of apoptosis genes.
In a second series of studies, Anders et al. evaluated clinically annotated gene expression data from over 700 early-stage breast cancers within two age-defined cohorts (≤45 years and ≥65 years of age). In this analysis, genomic expression profiling demonstrated significantly lower total mRNA levels of ERα, ERβ, and PR, with higher mRNA levels of both Her2/neu and epithelial growth factor receptor (EGFR, Her1) [48]. In a non-subtype-specific manner, Gene Set Enrichment Analysis (GSEA) revealed 367 significant gene sets enriched among young women’s tumors distinguishing them from tumors arising in older women [48]. Representative gene sets included those involved with immune function, hypoxia, BRCA1, stem cells, apoptosis, histone deacetylase, and multiple, targetable oncogenic signaling pathways, including Myc, E2F, Ras, and mTOR [48].
Following their initial analysis, the authors hypothesized that (1) breast cancer arising in younger women was enriched for more aggressive subtypes and (2) age-specific biologic differences were highly subtype dependent [56]. Additional analyses, including those from an independent microarray breast tumor data set, illustrated that younger women (age ≤45 years) as compared to older women (age ≥65 years) were more commonly diagnosed with basal-like and Her2-enriched breast tumors as defined by the PAM50 [57]. Moreover, when comparing breast tumor gene expression by age alone, hundreds to thousands of genes were differentially expressed; however, when correcting for subtype and other significant clinicopathologic features (i.e., grade), gene expression differences were no longer seen.
One recent study identified age-dependent expression of individual genes, even after correction for tumor subtype and grade. BUB1, KRT5, and MYCN were overexpressed in women under age 40 compared to older women, and CXCL2 underexpressed in young women (p ≤ 0.05). Within tumor subtypes, gene expression appeared to have an age-related impact on outcome. High levels of cytokeratin 5 and cytokeratin 6 expression were associated with inferior disease-free survival for young, but not older, women with basal-type (triple negative) and HER2-enriched breast cancers. Overexpression of ANGPTL4 predicted inferior disease-free survival in young, but not older, women with basal-type tumors [58].
Collectively, this series of analyses illustrates that while biological differences are clearly seen between breast carcinomas arising in a younger vs. an older host, intratumoral differences may be driven in large part by the higher distribution of aggressive subtypes (i.e., basal-like and Her2 enriched) among young women. The cause for the overrepresentation of more aggressive subtypes and the impact the pre- versus postmenopausal microenvironment on breast tumor biology have yet to be fully elucidated and are worthy of additional research.
8.4 Treatment and Management
Although the general principles of managing invasive breast cancer in young women are the same as in older women, there are a number of management and therapeutic issues requiring special consideration.
8.4.1 Early-Stage Disease
For many reasons, including lactation, sexual function, body image, and quality of life, breast-conserving surgery, whenever possible, is desirable for most young women. However, one of the most important risk factors for local recurrence after breast-conserving surgery is age <35 years at presentation [59]; these patients were found to have a nine times higher risk of local recurrence after conservative surgery than patients over 60 years [60]. Nevertheless, no studies have demonstrated conservative surgery in young women to have a negative impact on survival, so breast conservation remains appropriate for many. Breast conservation surgery should always be followed by radiation in young women, and adjuvant radiation is more often considered after mastectomy for young patients [61]. Patients who carry deleterious BRCA mutations (which are more common in those diagnosed with breast cancer at a young age) have been hypothesized to harbor a greater risk of radiation-induced malignancies, but data are limited [62].
In addition, young women are at heightened risk for distant recurrence, so adjuvant systemic therapies (e.g., chemotherapy and endocrine therapy) are usually warranted [63]. Young women are more likely than older women to have ER-negative tumors, which benefit more from chemotherapy. Adjuvant chemotherapy for early breast cancer in patients under 50 years old reduces the relative risk of recurrence by 35 % and of death by 27 % [64]. The choice of whether or not to give chemotherapy, and what regimen to give, is based more on stage and receptor status (i.e., clinical subtype) of disease than on age. However, it is important to recognize that the use of adjuvant therapies in young women can cause unique long-term side effects, including the induction of an early menopause, and associated fertility impairment, bone loss, and menopausal symptoms. Reductions in risk of recurrence with more aggressive therapies must be weighed against associated quality of life impairments and fully discussed with patients.
For young women with ER-positive early-stage breast cancers, adjuvant endocrine therapy reduces the risk of recurrence by approximately half [65]. Until recently, 5 years of tamoxifen was standard endocrine therapy for premenopausal early-stage breast cancer patients, but data from the ATLAS trial, aTTom trial, SOFT trial, and TEXT trial have revealed additional treatment options. ATLAS and aTTom found that 10 years of tamoxifen were superior to five [66, 67]. SOFT revealed that the addition of ovarian suppression to tamoxifen reduces risk of recurrence in patients under 35 and premenopausal patients who receive chemotherapy, and both SOFT and TEXT showed that treating these same patients with ovarian suppression and an aromatase inhibitor reduces risk of recurrence to an even greater degree [68, 69]. These findings are consistent with older studies showing that both relapse-free and overall survival are worse in patients who do not become amenorrheic following chemotherapy [70–73]. However, the SOFT trial data did not convincingly show that patients over age 35 who did not receive chemotherapy benefit from ovarian suppression. There may be a real, but small, risk reduction from ovarian suppression (with either tamoxifen or aromatase inhibitor) in some higher-risk patients aged 35–40 even if the disease was not biologically aggressive enough to warrant chemotherapy. On the contrary, it is important to consider the impact of ovarian suppression on physical and emotional health for very young women, including menopausal symptoms, insomnia, depression, and bone loss. Additional research is warranted to identify strategies to minimize the toxicities of endocrine therapy in young women diagnosed with breast cancer.
8.4.2 Metastatic Disease
Metastatic breast cancer in young patients is treated palliatively, with sequential systemic therapy regimens given until time of progression, just as in older patients. One caveat is that premenopausal patients with advanced ER-positive disease should additionally be treated with either bilateral salpingo-oophorectomy at the time of diagnosis with metastatic disease or receive an ovarian-suppressing medication (i.e., gonadotropin-releasing hormone (GnRH) agonist) throughout their subsequent endocrine therapies (including tamoxifen, aromatase inhibitor, fulvestrant, etc.) [74–76].
8.5 Outcomes
8.5.1 Age Disparities
AYA women diagnosed with breast cancer experience worse cancer-specific survival and relative survival compared to all other age groups below age 75 (Fig. 8.7). Young women had a worse survival from breast cancer compared to other cancers; in older age groups, breast cancer survival was superior to survival from other cancers. Breast cancer survival rates are lower for AYA women compared to older women, both cumulatively and stage by stage (Fig. 8.8) [1, 77]. The lowest overall rate of breast cancer survival for females diagnosed during 2000–2012 was in those aged 15–39 years, with 5-year rates of 81–88 %. In contrast, breast cancer survival for women between age 45 and 75 was 90–93 % (Fig. 8.7).
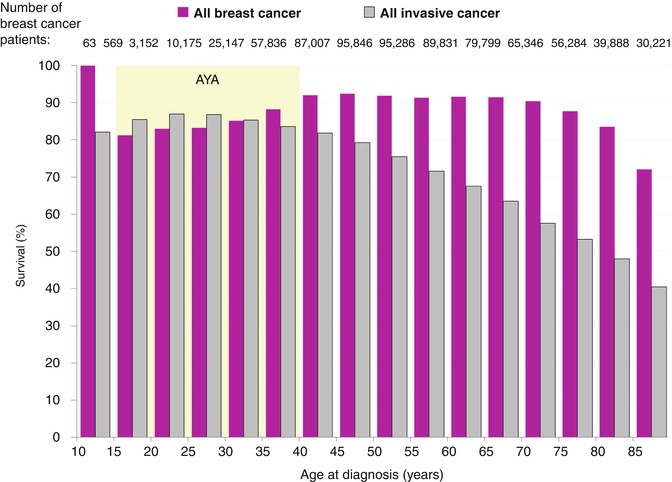
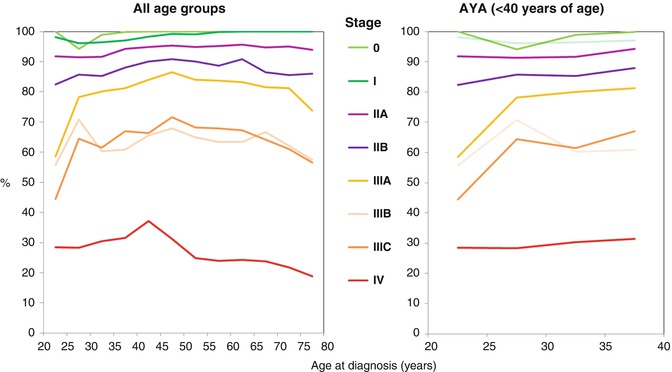

Fig. 8.7
5-year breast-cancer-specific survival in females, 2000–2012, SEER18

Fig. 8.8
5-year relative survival of women with breast cancer by stage at diagnosis, AJCC6 (all eight categories), 2004–2010, SEER18
The age-related disparities in breast cancer survival vary by stage at diagnosis, although survival is inferior for AYAs compared to 40–50-year-old women at all stages of breast cancer (Fig. 8.8). Above the age of 40, stage 0 in situ breast cancer has a 100 % 5-year relative survival, even in elderly women. For all age groups with stage 0 disease, only AYAs have less than 100 % 5-year survival. For stage I breast cancer, women greater than 50 years of age have the highest survival rates and 25–35-year-olds have the lowest survival rates. Women with stage II–IV cancer who are 40–50 years of age have the best prognosis, with comparatively inferior prognosis for AYA women. Stage IV breast cancer has a far worse prognosis than any other stage. Within the AYA age group, survival is inversely proportional to age for all stages of breast cancer, with the most inferior survival rates occurring in the youngest AYA women (Fig. 8.8).
Most histologic subtypes have a worse prognosis in AYA women than in older women. The 5-year relative survival for AYA was also inferior for women with each histologic subtype of breast cancer, including infiltrating ductal, medullary, lobular, and inflammatory breast cancer, as well as Paget’s disease of the breast (SEER 18 data, not shown) [78]. The outcome disparity between age groups by histologic subtype is most apparent for invasive ductal carcinoma (p < 0.001, SEER 18 data, not shown).
Since 1985, the rate of survival improvement has been greater in AYA women than in older women for all stages at diagnosis (Fig. 8.9). The rate of survival improvement in AYA women has been directly proportional to the extent of disease at diagnosis, with the greatest relative gains for women with distant disease at diagnosis (Fig. 8.9). Thus, the discrepancy in survival between younger and older women is narrowing over the last three decades, possibly due to increasing clinical focus on the distinctive health-care needs of young women with breast cancer and to more effective palliative and supportive care strategies for previously healthy women diagnosed with advanced disease.
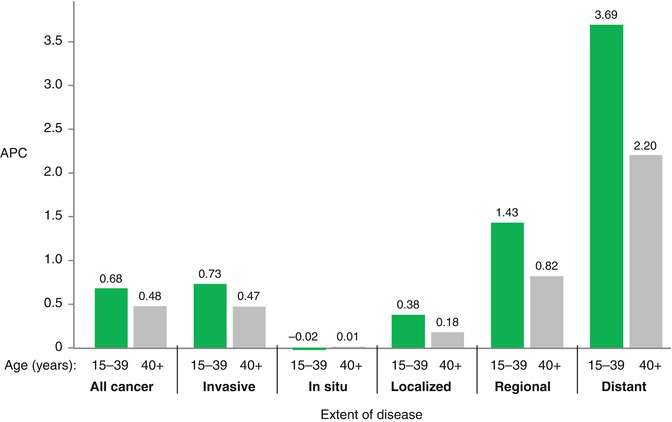

Fig. 8.9
Annual % change (APC) in 5-year relative survival of women with breast cancer by age and extent of disease at diagnosis, from 1985 when the impact of screening on incidence had stabilized, to 2006, SEER9
The recent Prospective Outcomes in Sporadic versus Hereditary breast cancer (POSH) study examined breast cancer outcomes for British women diagnosed at age 18–40 years of age at diagnosis. Young patients had similar and inferior outcomes regardless of hormone receptor expression, with the incidence of relapse peaking at 2 years post-therapy for patients with ER-negative tumors and continuously declining over an 8-year period for patients with ER-positive tumors [79]. Patients with a positive family history of breast cancer were more likely to have high-grade tumors, but were not more likely to have an adverse outcome by multivariable analysis [80]. Obesity was associated with adverse biological features and was an independent risk factor for death from breast cancer [81].
8.5.2 Racial Disparities in AYA Women
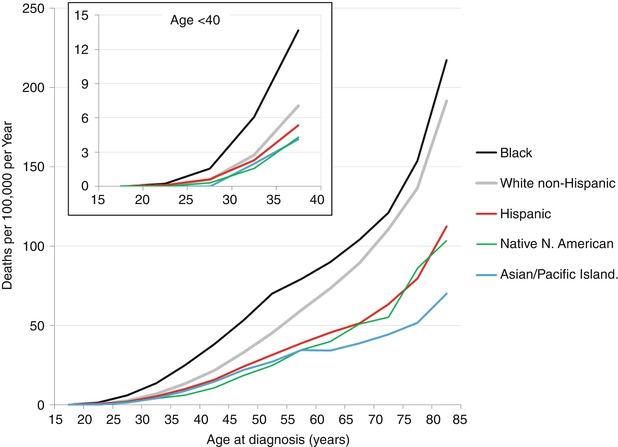
In women <40 years of age (as in all age groups), African Americans and Native North Americans have the worst survival, and Asians have the highest survival rates. Young African American women have disproportionately high breast cancer mortality in comparison to other racial groups (Fig. 8.10). One study found that black women <40 had larger tumor size, higher rates of local and distant metastasis, a higher proportion of ER negativity, and a higher rate of medullary tumors. Relative risk for death was 1.94 for localized disease, 1.58 for regional disease, and 2.32 for metastatic disease compared to white women [82]. Another study showed that young black women with stages III and IV disease had worse prognosis despite standard therapy [82]. In the POSH study, black race was associated with adverse biological features and with adverse outcome despite equal access to health care, with 71.1 % 5-year OS in black compared to compared to 82.4 % in white patients (P = 0.0160) [83]. Because 10 % of black women (vs. 5 % of white women) with breast cancer are diagnosed prior to age 40 and because young black women have worse outcomes, they are considered a particularly high-risk group [84].