23 Breast Cancer and Pregnancy
Epidemiology
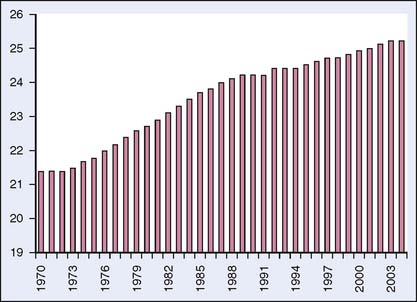
An estimated 192,500 cases of breast cancer were diagnosed in U.S. women in 2009.1 Of these, an estimated 0.2% to 3.8% of breast cancers were in pregnant women,2 which is approximately 1 in 3000 pregnancies.3 Increasing age is a risk factor for breast cancer; thus the incidence of breast cancer during pregnancy is expected to increase as women delay childbearing. Data from the Centers for Disease Control and Prevention (CDC) show that the birth rate for older women continues to rise4 (Fig. 23-1). The birth rate for women aged 35 to 39 years in 2004 was 45.4 per 1000 women, a 4% increase from the previous year. For women 40 to 44 years of age, the birth rate in 2004 was 8.9 per 1000 women, a 2% increase. For this same time period, the birth rate for women 15 to 34 decreased by 1%. Concomitant breast cancer and pregnancy may present difficulties for the medical team, since treatment of breast cancer may compromise the developing fetus, yet inadequate treatment of the breast cancer may portend a poor outcome for the mother.
Presentation
Pregnancy-associated breast cancer (PABC) is defined as breast cancer occurring in pregnancy or within 1 year postpartum. Breast cancer during pregnancy typically presents as a painless mass or thickening in the breast.5 Series of pregnant women with breast cancer from the Mayo Clinic and from Britain showed that 60 of 63 (95%) and 146 of 178 (82%) women, respectively, presented with a painless mass.6,7 A thorough breast examination early in pregnancy is critical. As pregnancy progresses, the breast tissue undergoes physiologic changes, including increasing firmness, nodularity, and hypertrophy, which may obscure a mass.8 Combined with a low suspicion of malignancy in younger patients, these factors may lead to a delay in diagnosis and treatment, which recent studies estimate at 1 or 2 months.9 For example, in a study from Memorial Sloan-Kettering Cancer Center, only 12 of 56 women with breast cancer during pregnancy were diagnosed before delivery.10 This delay can lead to more advanced-stage disease at diagnosis. A 1-month delay in treatment of an early-stage breast cancer with a doubling time of 130 days increases the risk of axillary node metastases by 0.9%. A 3-month delay increases the risk by 2.6%, and a 6-month delay by 5.1%. With a doubling time of 65 days, the risks are doubled.11
Pathology
Most cases of breast cancer in pregnant women are invasive ductal carcinoma, with invasive lobular carcinoma diagnosed infrequently. Most tumors are high grade, and lymphovascular invasion is common.12 In one prospective study of 39 patients with breast cancer diagnosed during pregnancy, only 28% were estrogen receptor (ER)-positive and 24% were progesterone (PR)-receptor positive compared with 45% and 36%, respectively, of nonpregnant young women with breast cancer.12 These results concur with the suggestion that hormone receptor-positive disease is age-related and is diagnosed more often in postmenopausal women. It has been hypothesized that high circulating levels of estrogens may even prevent ER-positive cancers.13
HER2/neu overexpression in PABC has been evaluated in a small number of case studies. In their study of PABC, Elledge and associates14 noted that 7 of 12 patients (58%) overexpressed HER2/neu. A report by Middleton and associates12 from M.D. Anderson did not find any difference in the HER2/neu expression rate (28%) in pregnant versus nonpregnant young women with breast cancer.
Recent reports seem to dispute the historical idea that inflammatory breast cancer was more common in pregnancy.15 Indeed, data published since the 1960s have shown a similar incidence of inflammatory breast cancer in both pregnant and nonpregnant patients, ranging from 1.5% to 4.2%.9
Overall, these results suggest that the histopathologic and immunohistochemical properties of breast cancer in pregnant women are similar to those found in nonpregnant young women. It appears likely that age at diagnosis, rather than pregnancy, determines the biologic nature of the malignancy.12
Imaging
As in nonpregnant women, most breast masses detected during pregnancy are benign. However, a persistent or clinically suspicious mass in the breast and/or axilla should be imaged and biopsied, especially if imaging fails to suggest a benign etiology for the abnormality.16
Using modern equipment and shielding, mammography should pose little risk to the developing fetus. Standard bilateral mammography delivers a dose of 200 to 400 mGy. With appropriate shielding, the fetus is exposed to less than 50 mrad of radiation, which is well below the level of 10 rad (100 mGy) that increases the risk of fetal malformations by 1% and is also less than the estimated environmental exposure of 2 mGy per week.17
Although it is considered safe, some controversy has surrounded the efficacy of mammography during pregnancy. As aforementioned, the breast undergoes physiologic changes during pregnancy, including increased density and nodularity, which may make interpretation of the mammogram difficult. In a review of cases by Max and Klamer18 between 1970 and 1980, 6 of 8 pregnant women with breast cancer had normal mammograms. In contrast, a more recent study reported that 18 of 23 mammograms in patients with PABC were abnormal.19 A 2003 report by Ahn and colleagues20 evaluated the mammograms of 15 patients with breast cancer diagnosed during pregnancy. Despite all the patients having dense breasts, 13 had abnormal mammographic findings. In addition to identifying masses in eight patients, other abnormal findings included calcifications, axillary adenopathy, diffuse skin and trabecular thickening, and asymmetric densities. The authors concluded that even though increased breast density may obscure masses in a pregnant patient, there are other suspicious findings that can be identified.20
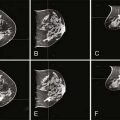
Ultrasonography is a safe and relatively sensitive method of imaging breast masses in pregnant patients. In a study by Liberman and colleagues,19 6 of 6 patients with PABC had a focal solid mass on ultrasound.19 In their series, Ahn and colleagues20 reported that 19 of 19 patients with PABC had a solid mass on ultrasound. It is interesting that the ultrasound findings in Ahn’s report differed somewhat from ultrasound findings in non-PABC in that the investigators observed posterior acoustic enhancement and a marked cystic component in the PABC cases. Despite the disparity, they concluded that ultrasound is a highly useful imaging technique in PABC.20
The use of breast magnetic resonance imaging (MRI) in pregnant patients has not been thoroughly evaluated. Even though MRI has been used to evaluate fetal anomalies and appears safe in the second and third trimesters, it cannot be recommended in the first trimester because its effects on fetal organogenesis are unknown.21 In addition, the use of gadolinium contrast is not recommended because it is known to cross the placenta and has been shown to cause developmental, skeletal, and visceral abnormalities in rats and because it is classified as a pregnancy category C drug.21,22 Of interest, Talele and associates23 reported a case of a lactating woman with a palpable breast mass in whom mammography and ultrasonography failed to reveal a mass. The patient then underwent MRI of the breast, which showed increased gadolinium uptake throughout the breasts, and in several areas the uptake was similar to that seen in cases of breast cancer. An excisional biopsy was performed and the pathology revealed benign changes consistent with lactation. The authors concluded that increased vascular permeability associated with lactation alters gadolinium uptake in the lactating breast and may mimic malignancy. Further study of the role of MRI in evaluating suspicious masses in the lactating breast was recommended.23
For the detection of metastatic disease, MRI is preferable to ultrasonography for imaging the liver if the patient is in the second or third trimester. MRI is also the safest and most sensitive method for imaging the brain.24 CT scanning of the abdomen and pelvis is avoided during pregnancy because of the risk of fetal exposure to radiation. Bone scans can be done safely during pregnancy if there is adequate hydration and a urinary catheter is placed for 8 hours to avoid retention of radioactivity in the bladder.25 For patients with no complaints suggestive of bony metastases outside the spine, a screening MRI of the thoracic and lumbosacral spine may be preferable.26 Chest radiography is considered safe in pregnancy with estimated fetal exposure of 0 to 10 mrad. The exposure to the fetus is higher with portable x-ray films than with standard equipment.9
Biopsy
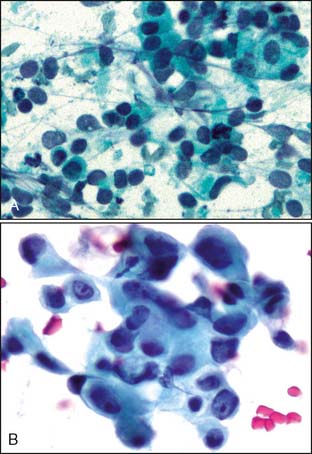
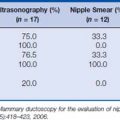
Pathologic diagnosis should be accomplished using the least invasive technique that will provide a definitive answer. In the setting of PABC, core needle biopsy has advantages over fine-needle aspiration (FNA) because it provides tissue for histopathologic study as well as determination of hormone receptor- and HER2/neu status. There has been concern over the development of a milk fistula as a complication of a core biopsy. However, this idea appears to have been overestimated with only one documented case reported in the literature.27 Direct smears showing pregnancy-related changes in breast tissue versus primary breast cancer in pregnancy are shown in Figure 23-2.
Staging
Since recommendations on therapy hinge on disease extent, accurate staging of PABC is crucial to the formulation of a reasonable treatment plan. Depending on the clinical stage at diagnosis, a complete staging evaluation should be undertaken, including chest x-ray with abdominal shielding, liver ultrasound, and screening noncontrast MRI of the spine to exclude bone metastases, if clinically indicated.16 The use of CT scans should be discouraged.
Treatment
The optimal treatment for breast cancer includes a multimodality approach, including surgery, chemotherapy, and radiation (Fig. 23-3). For PABC, a similar strategy can be used, although radiation therapy is generally contraindicated in pregnancy owing to the putative risks to the fetus.
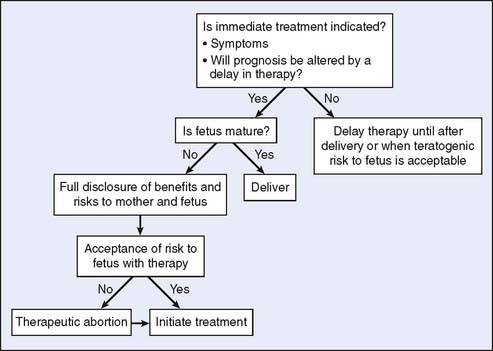
Figure 23-3 Cancer in pregnancy: approach to treatment. RT, radiation therapy.
(From Wooldridge JE, Doll DC: Special issues in pregnancy. In Abeloff JO, Armitage JE, Niederhuber MB, et al (eds): Clinical Oncology, 3rd ed. Philadelphia: Elsevier, 2004, p 1336.)
Surgery
As in the nonpregnant patient with breast cancer, surgical resection is the definitive treatment for PABC and should not be delayed because of pregnancy.9 Nonobstetric surgery and anesthesia during pregnancy can be done without significant risk to the fetus.28 Byrd and associates29 performed breast biopsies on 134 pregnant women and reported only one miscarriage. Mazze and Kallen30 found an increased risk of low and very-low-birth-weight infants in women who underwent surgery while pregnant, but the authors suggested that it was the under-lying illness requiring surgery, rather than the surgery itself, that led to the low birth weights.
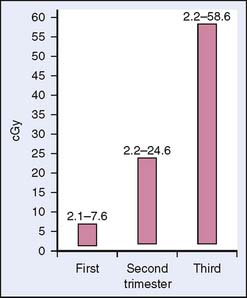
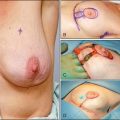
Mastectomy and axillary dissection are considered the optimal choice for stages I and II and some stage III tumors diagnosed in the first or second trimester when the patient desires to continue the pregnancy.5 In nonpregnant patients, breast-conserving surgery (lumpectomy and axillary lymph node dissection) followed by radiation has been shown to be as effective as modified radical mastectomy for localized disease.31 However, breast irradiation during pregnancy is relatively contraindicated because of concerns of excessive radiation exposure to the fetus, which could cause congenital abnormalities or an increased risk of malignancy in the child (Fig. 23-4). The estimated radiation dose to the fetus from breast radiation therapy depends on the stage of pregnancy17 (Fig. 23-5). Mastectomy, which eliminates the need for postoperative radiation, is the recommended treatment.
Axillary dissection is preferred since the axillary nodal status has a significant impact on adjuvant therapy and because sentinel node evaluation poses an unknown risk. It has been suggested that sentinel node biopsy with a minimal dose of 500 to 600 μCi using double-filtered technetium-Tc99 (99mTc)-sulfur colloid is safe.24 After a dose of 0.1 mCi of 99mTc-sulfur colloid for sentinel node mapping, Pandit-Taskar and associates32 estimated a fetal absorption of 0.00014 cGy, an amount well below the National Council on Radiation Protection and Measurements limit for a pregnant woman. Mondi and associates33 reported on nine pregnant women (three with breast cancer, six with melanoma) who underwent sentinel node mapping. There were no adverse reactions to the sentinel lymph node, and all patients had term deliveries of normal infants Although early reports suggest that sentinel node mapping in pregnant patients is safe, there are, however, no supporting studies for this procedure at this time.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree