Etiology
Prevention
Early detection
Follow-up
Treatment
Survivorship
• Biology and
• Chemo-prevention
• Mammography
• Access
• Adjuvant chemotherapy
• Treatment side effects
• Risk factors
• MRI
• Quality
• Radiation therapy
• Adherence to adjuvant hormone therapy
• Self-exam
• Timeliness of therapy
• Surgery
• Coping skills
• Body image
• Social support
• Acculturation
• Quality of life
Table 4.2
Average annual (2006–2010), age-adjusted invasive female breast cancer incidence and mortality rates per 100,000 women, and 5-year relative survival probabilities among race groups and Hispanics/Latinos
Population | Incidence rate (per 100,000) | Mortality rate (per 100,000) | 5-year survival probability (%) |
|---|---|---|---|
White | 127.4 | 22.1 | 90.4 |
African-American | 118.4 | 30.8 | 78.9 |
Asian/Pacific Islander | 84.7 | 11.5 | 91.4 |
Hispanic/Latino | 91.1 | 14.8 | 87 |
American Indian/Alaska Native | 90.3 | 15.5 | 85.4 |
Disparities Across the Cancer Control Continuum
Etiology and Prevention
An examination of risk factors for poor-outcome disease can provide some clues to these disparities. For example, modifiable risk factors, e.g. obesity, pregnancy, and postmenopausal hormone use, are differentially distributed among populations where we see disparities, e.g. African American and Hispanic women are more likely to be overweight/obese vs. white women, however, most of the literature in this area has provided inconclusive results. Furthermore, what we know about the uptake of chemoprevention, diet, exercise and prophylaxis, including genetic testing and surgery, in most of these minority populations is lagging behind that of white women as well as women residing in more urban, as opposed to rural, areas. Studies of non-modifiable risk factors, e.g. genetics, have provided more conclusive results. Germline mutations, for example, BRCA1 mutations, result in higher risk for triple negative cancers (American Cancer Society 2013). Despite advancements in genetic testing leading to reductions in morbidity and mortality associated with breast cancer, research suggests that African American and minority women are significantly less likely to receive genetic counseling and testing in comparison to white women (Howlander et al. 2014). Health care reform now requires that insurance cover the cost of genetic testing. However, for populations of women who are not covered by health insurance, genetic testing is incredibly expensive, typically costing around $3,400. Exorbitant costs associated with genetic testing clearly place minority and impoverished women at a certain disadvantage for breast cancer outcomes (Hall and Olopade 2006; Johns Hopkins Medicine Breast Center; Susan G. Komen Testing for BRCA1 & BRCA2 Mutations).
Triple Negative Breast Cancer (TNBC), accounts for 10–20 % of invasive BC, but has poorer prognosis than luminal tumors and treatment options are more limited (Boyle 2012). Risk of TNBC is roughly three times higher among non-Hispanic black women and pre-menopausal women (Boyle 2012). Moreover, a study from Ghana found that there might be a genetic predisposition to TNBC among women of African ancestry (prevalence of TNBC 82 % in Ghana, 33 % among African American women and 10 % among white American women) (Boyle 2012). Similarly, among Asian/Pacific Islander women, the risk of ER/PR positive tumors is higher among Korean women vs. Hawaiian women (Li et al. 2002). Both Hispanic and American Indian/Alaskan Native women have larger tumors and more advanced disease at diagnosis (Mejia de Grubb et al. 2013; Von Friederichs-Fitzwater et al. 2010).
Screening Behavior
While guidelines have changed over time, clearly average-risk women over age 50 should have a mammogram every 2 years; moderate to high risk women should consult with their physician as to when to start screening, what modality (MRI vs. mammography) and how often. In addition, access to high quality imaging services and prompt/proper follow-up of abnormalities found should be available to all populations. Access to breast cancer screening as well as differences in quality of care among black and white women have contributed greatly to observed disparities in breast cancer mortality. For example, from 1999 to 2003 in Chicago, the mortality rate for breast cancer was 49 % higher among black women compared to white women. In 2003, the mortality rate increased to 68 % higher among black women compared to white women (Hirschman et al. 2007). Explanations for this observed disparity have focused on gaps in education, access to screening, as well as differences in quality of care between black and white women. Research suggests that white women in Chicago are more likely than black women to attend academic and private healthcare facilities, as well as more likely to have their mammograms read by specially trained radiologists (Ansell et al. 2009).
Disparities in mammography use are hard to determine because of the reliance on self-reported use in the most commonly used metric for screening utilization, the Behavioral Risk Factor Surveillance System (BRFSS). Self-reports of recent mammography use are actually highest among African-American women (77 %) than white women (75 %), however, verification of self-reports drop these rates to 59 % vs. 65 %, respectively [Frieden and Centers for Disease Control and Prevention (CDC) 2012]. For example, in Chicago, self reported mammogram screening rates have been similar for blacks and whites since 1996 despite the dramatic difference in breast cancer mortality rates. However, poor and black women tend to over-report screening by as much as 30 % (Hirschman et al. 2007). Asian Pacific/Islander women in general have a 74 % prevalence rate (self-reported); however, disparities exist in mammography prevalence among Asian subgroups e.g. South Asian women (40 %) vs. Japanese Women (71 %) (Frieden and Centers for Disease Control and Prevention (CDC) 2012; Lee et al. 2002). For American Indians/Alaskan Natives (AI/AN), rates are at 69 %, and geographical differences have also been noted, i.e. AI/AN women from Alaska had higher screening rates than those living in the Southwest [Centers for Disease Control and Prevention (CDC) 2012; Schumacher et al. 2008]. Hispanic women also report moderate screening rates, 70 %; compared to non-Hispanic women (Lim et al. 2009; Lopez-Class et al. 2011; Native American Cancer Research Corporation Native Americans and Cancer). The women from these other racial/ethnic groups are more likely to face cultural barriers to receiving screening, e.g. prefer traditional holistic medicine to Western medicine or have modesty concerns. Ultimately, no single intervention will have the ability to reduce mortality associated with breast cancer in disparity stricken areas such as Chicago. Only through a multifaceted approach that addresses issues such as cultural differences, increased health education, access to care and decreasing barriers to screening, will the mortality gap begin to narrow.
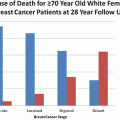
Stage at Diagnosis
Stage at diagnosis is an indicator of both quality of care (e.g. good mammography use and follow-up), as well as outcomes following treatment. Many studies have demonstrated that women living in rural areas are diagnosed at later stages compared to urban breast cancer patients (Monroe et al. 1992; Nguyen-Pham et al. 2014; Amey et al. 1997; Howe et al. 1992). Moreover, rural African-American women are diagnosed at later stages compared to rural white and urban white and African-American women (Amey et al. 1997). Asian women, on the other hand, are more likely to be diagnosed at Stage 1 compared to African-American women, American Indian/Alaskan Native, and Hispanic women; however, again within Asian subgroups there are disparities in not only stage of diagnosis, but age at diagnosis, and tumor grade (see Tables 4.3) (Li et al. 2002; Mejia de Grubb et al. 2013; Von Friederichs-Fitzwater et al. 2010; Yi et al. 2012).
Table 4.3
Average annual (2007–2011), age-adjusted invasive female breast cancer incidence rates per 100,000 women, percent late (regional and distant) stage at diagnosis, and 5-year relative survival probabilities according to metropolitan/non-metropolitan residence
Population | Incidence rate (per 100,000) | Percent late stage (%)a | 5-year survival probability (%) |
|---|---|---|---|
Metropolitan | 122.1 | 73.6 | 89.1 |
Non-metropolitan | 111.2 | 72.5 | 86.9 |
Follow-up for Abnormal Screening Tests
Prompt and proper follow-up for any abnormalities detected on screening is crucial to improving outcomes. Issues such as access (e.g. facilities, proper technology, insurance coverage, transportation), quality state-of-the-art facilities, proper testing, and competent providers are crucial to the receipt of follow-up care. Studies have documented longer intervals for follow-up after an abnormal mammogram for African-American women, even with similar insurance status, compared to white women [Centers for Disease Control and Prevention (CDC) 2012]. Language barriers also contribute to disparities in follow-up in non-English speaking women (Karliner et al. 2012; Austin et al. 2002; Janz et al. 2009; Sammarco and Konecny 2010; Nápoles et al. 2011; Yanez et al. 2011). Rural women are less likely to receive follow-up testing, probably due to lack of access and facility factors (Schootman et al. 2000; Goldman et al. 2013). Finally, a study among Medicare beneficiaries found that facilities serving more vulnerable populations had lower follow-up rates for women with abnormal screening tests.
Interventions to Address Disparities
Important policy interventions occurred in the 1990s to improve mammography use among vulnerable populations. First, the Centers for Disease Control and Prevention (CDC) started the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) which made free or low-cost mammograms available to low-income, under and uninsured women. Between 1991 and 2006, 1.8 million received breast cancer screening through the NBCCEDP (Hoerger et al. 2011). This program also provides follow-up care after abnormal testing and initial treatment. A recent analysis of data indicated that NBCCEDP is doing a good job in getting women in for timely and quality follow-up [Centers for Disease Control and Prevention (CDC) 2012]. One problem with NBCCEDP is that only a fraction (fewer than 20 %) of eligible women in the U.S. utilize NBCCEDP due to the funding caps on this program (NBCCEDP Breast Cancer Expert Panel 2005).
Secondly, the National Cancer Institute (NCI) developed the Cancer Control Plan, Link, Act, Network with Evidence-based Tools (PLANET) Research-Tested Intervention Programs (RTIPs) which stores research-tested interventions for improving the use of screening (including breast cancer screening) in underserved populations (Sood et al. 2007). For example, the North Carolina—Breast Screening Program (NC-BSP) and Forsyth County Cancer Screening Project (FoCaS) are two programs on RTIPs that provide interventions for improving breast cancer screening among African-American women (Earp et al. 2002; Paskett et al. 1999). Other RTIPs programs are available for Alaskan Native, American Indian, Asian, Hispanic, Pacific Islander and non-Hispanic White women. Programs are free to download; however, few data exist on the effect of diffusion and implementation of RTIPs.
Other successful interventions for reaching vulnerable populations for improving mammography use include utilizing patient navigators (PN) to reduce barriers such as modesty and cultural issues, and including spiritual and religious themes (Freeman 2006; Paskett et al. 2012). For women living in rural areas, mobile mammography (Gardner et al. 2012), free/reduced services (Lane and Martin 2012), as well as agents of change (e.g. lay advisors, PN, public health nurses) (Paskett et al. 2006) have proven successful to improve uptake of mammography. Funding to continue these efforts is a significant challenge.
Treatment
Disparities in treatment have been well-documented. African-American, American Indian and Hispanic women are less likely to have surgery, more likely to refuse surgery, and less likely to receive radiation therapy (RT) compared to non-Hispanic white women (Li et al. 2003). Women from Appalachia (a predominately rural area) have higher rates of mastectomy and lower rates of RT after breast cancer surgery compared to non-Appalachian women (Freeman et al. 2012). Women >70 years and women without insurance were also less likely to receive adjuvant RT (Freeman et al. 2012). Disparities also exist within Asian/Pacific Islander groups for receipt of surgery and RT (Yi et al. 2012). Quality of treatment is also a factor. Fewer African-American women start treatment within 30 days (69 %) compared to white women (82 %); and receive lower quality treatment (Centers for Disease Control and Prevention (CDC) 2012). This is a significant problem, as a modeling study estimated that up to 19 % of the mortality difference between African-American and non-Hispanic white women could be eliminated if the same treatment was provided to both groups (Centers for Disease Control and Prevention (CDC) 2012). Differences in response to treatments, such as Tamoxifen, may also be responsible for some of the disparities in outcomes (American Cancer Society 2013). This area needs to be further explored.
Issues of Survivorship
Issues of survivorship include side effects of treatment, adherence to adjuvant hormone therapy, coping skills, body image, social support, acculturation and quality of life. Hispanic women have been found to suffer more from pain, fatigue, depression, and financial hardship related to treatment compared to non-Hispanic women (Fu et al. 2009; Graves et al. 2012). American Indian/Alaskan Native women also report problems related to pain, fatigue, depression and hair loss (Burhansstipanov et al. 2010). Latina Spanish speaking women are more likely to discontinue adjuvant hormone therapy compared to white women (Livaudais et al. 2012).
Coping skills allow women to adjust to both physical and emotional distress during and following a cancer diagnosis and treatment. There are significant ethnic, racial and cultural differences in coping strategies used to respond to these stressors. For example, positive and negative forms of coping were more common among women of color than white women; negative coping was more likely to be associated with increased levels of distress and poorer survival (Yoo et al. 2014). Rural breast cancer patients are more likely to use behavioral disengagement, which is related to depressive symptoms compared, to urban patients (Schlegel et al. 2009; Collie et al. 2005).
Factors significantly related to coping strategies, such as religion and spiritual practices, are actually more relevant for minority and rural women. Some practices, e.g. spirituality and family support, actually are helpful in African-American populations, whereas spirituality has little impact on most non-Hispanic white women or negative effects in Asian/Pacific Islander, Hispanic, and American Indian/Alaskan Native women (Austin et al. 2002; Gaston-Johansson et al. 2013; Ashing-Giwa et al. 2013a; Daley et al. 2012; Ndikum-Moffor et al. 2013).
Body image and femininity are domains often impacted by breast cancer diagnosis and treatment. Most studies have been conducted among African-American women, and indicate that body image concerns were very important to their treatment decisions (Yoo et al. 2014; Hawley et al. 2009). Asian/Pacific Islander women report negative feelings towards their bodies after cancer surgery, so much so, that they report loss of self-worth, unhappiness and depression, and avoid looking at their bodies in the mirrors (Ashing-Giwa et al. 2013a). American Indian/Alaskan Native women associate hair loss due to chemotherapy as a sign of loss of spiritual strength which could result in isolation from the tribe (Burhansstipanov et al. 2010).
Social support is seen different in vulnerable populations—more African-Americans report receiving social support from God whereas non-Hispanic whites report receiving support from family and friends (Gaston-Johansson et al. 2013). In Asian culture, women are seen as nurturers not dependents, thus Asian breast cancer survivors may have unmet social support needs (Ashing-Giwa et al. 2013a). Latina women report the family as the main source of social support, however, with a breast cancer diagnosis, women report less acceptance by their husbands, possibly due to a change in gender roles and perceived femininity, resulting in lower perceived social support (Lopez-Class et al. 2011; Ashing-Giwa et al. 2004).
Acculturation also impacts survivorship. Lower acculturated Latinas report poorer health after breast cancer and more functional limitations and poorer mental health (Janz et al. 2009; Sammarco and Konecny 2010; Nápoles et al. 2011; Yanez et al. 2011). Native American languages have no word for cancer, but it translates to “the disease for which there is no cure.”(Native American Cancer Research Corporation Native Americans and Cancer) Language barriers compound acculturation issues and produce long-lasting problems with access and adherence (Graves et al. 2012; Ashing-Giwa et al. 2013b).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree