Lodovico Balducci, Dawn Dolan, Christina Laronga
Breast Cancer
Approximately 35% of breast cancers occur in women aged 70 and older, and this percentage is expected to increase with the aging of the population.1 Age is the most important risk factor for breast cancer.
Breast cancer in the aged is by and large a chronic disease that involves individuals affected by other chronic health conditions, including polymorbidity, polypharmacy, functional dependence, and geriatric syndromes.2 The primary care provider is pivotal in coordinating the care of these patients, in addressing barriers to treatment, in supporting the home caregiver, and in managing the long-term therapeutic complications. The management of breast cancer in the older woman should be based on individual life expectancy and tolerance of treatment. Personalized treatment plans may require the input of various professionals, including nurses, pharmacist, social worker, and dietician in addition to different medical specialists.
In this chapter we review the basic principles of breast cancer management, the epidemiology of breast cancer in the older women, and the age-specific questions related to the treatment of breast cancer.
Principles of Breast Cancer Management
Only the management of cancer in the postmenopausal woman is reviewed in this chapter, as the management of the premenopausal woman may involve ovarian suppression that is not pertinent to the older individual. The management is determined by the pathology and the stage of the disease, as well as by the patient’s life expectancy and treatment tolerance.
Pathology
The most common histologic forms of breast cancer include ductal carcinoma and lobular carcinoma.3 Lobular carcinoma is more frequently bilateral than ductal carcinoma. Both forms of cancer may be invasive or in situ. Ductal carcinoma in situ (DCIS) and lobular carcinoma in situ (LCIS) have minimal risk of metastatic disease but may become invasive carcinomas and spread to other organs if left untreated. With the widespread use of mammography, DCIS and LCIS may account for as many as 30% of newly diagnosed breast cancer cases.4 The value of diagnosing these conditions in individuals with limited life expectancy has been questioned, because the evolution to invasive disease may take several years.
A number of histologic characteristics, including the histologic and nuclear grade and the proliferation rate (commonly assessed by the presence of Ki-67), indicate the aggressiveness of breast cancer and the risk of metastatic spread but have little influence on the treatment choice.3
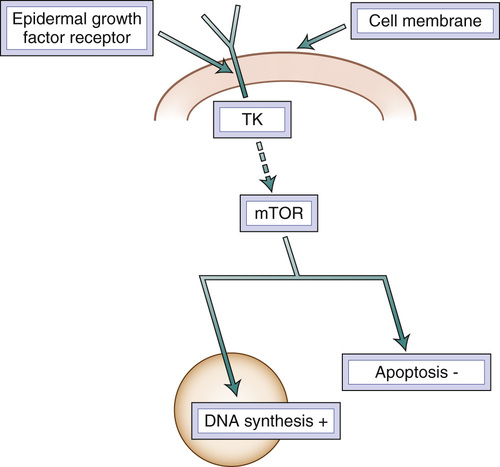
All newly diagnosed breast cancers should be assessed for the presence of estrogen and progesterone receptors and for overexpression of human epidermal growth factor receptor 2 (HER2).3 In the absence of hormone receptors, hormonal treatment is not effective. Approximately 15% to 25% of breast cancers have HER2 membrane receptor overexpression, which maintains the independent growth of breast cancer. HER2 is part of the family of epidermal growth factor receptors that are present in normal breast. These have an extracellular, a transmembrane, and an intracellular component, consisting of a tyrosine kinase (Figure 86-1). This enzyme, activated by a receptor-generated signal, initiates the transduction cascade that leads to DNA synthesis, cell proliferation, and apoptosis inhibition. A number of monoclonal antibodies and tyrosine kinase inhibitors effectively treat HER2 overexpressing tumors. The so-called triple-negative breast cancers (those in which neither estrogen receptors nor progesterone receptors nor HER2 is expressed) represent the most aggressive forms of breast cancer and those for which the systemic treatment is least effective.
Analysis of the breast cancer genome has allowed clinicians to fine-tune the systemic treatment and, in particular, to identify those patients with hormone receptor–rich tumors for whom chemotherapy may add additional benefit.5 The genome analysis may also indicate which cytotoxic agents are more promising for treating different types of breast cancer.
Breast Cancer Treatment
The discussion of the staging of breast cancer is very complicated, of limited interest to a primary care or geriatric practitioner, and beyond the scope of this chapter. In describing the management of breast cancer we will refer to localized, locally advanced, and metastatic disease. The management of breast cancer at early stages may involve both local and systemic treatment.3 Local treatment includes total mastectomy, partial mastectomy with breast preservation, axillary lymph node dissection, and radiation therapy. Nowadays a full axillary lymph node dissection is performed only in individuals who test positive for cancer in the sentinel lymph nodes (SLNs) or those rare cases in which the SLNs may not be identified. SLNs are the first nodes to receive the lymphatic drainage from the breast tumor and may be recognized by mapping of the axilla with radioisotopes or blue dye.
Radiation therapy may be administered to the breast after partial mastectomy to prevent local recurrences and to allow breast preservation. Intraoperative radiation therapy prevents the inconvenience of daily visits to the radiation therapy suite. It may also be administered to the chest walls and to the axilla in patients at high risk of local recurrence after total mastectomy and axillary dissection. These include patients whose primary tumor was larger than 5 cm and those with involvement of four or more axillary lymph nodes. It is debated whether all patients who have had total mastectomy may benefit from postoperative radiation therapy to the chest wall.6,7 Radiation therapy may also obviate the need for axillary dissection and ameliorate its complications in patients with SLNs involved by the tumor.8 Radiation therapy is also used for management of metastases in the brain, which may be unreachable by systemic treatment, and for palliation of metastatic disease, such as bone pain.3
Systemic therapy of breast cancer involves hormonal, cytotoxic, and biologic agents (Box 86-1). Selective estrogen receptor modulators (SERMs) are currently seldom used in the United States because they may be less effective than the aromatase inhibitors (AIs) and may cause, albeit very rarely, deep vein thrombosis and endometrial cancer.9 Unlike the AIs, the SERMs prevent osteoporosis and do not cause unfavorable changes in lipid profiles. The AIs include steroidal (exemestane) and nonsteroidal (letrozole, anastrozole) drugs.9 The effectiveness and the risk of complications of various AIs appear the same. The most serious complications include osteopenia and osteoporosis. It is advisable to obtain a baseline dual-energy x-ray absorptiometry (DXA) scan before instituting the treatment and to make sure that older patients are taking calcium and vitamin D supplements even if the DXA scan is normal. In two studies, osteoporosis was prevented by the prophylactic administration of zoledronic acid monthly and every three months,10,11 but it is not clear whether such intensive regimen is really necessary. Although AIs may cause an increase in cholesterol and low-density lipoprotein (LDL) concentration, it has never been conclusively demonstrated that AIs cause increased risk of coronary artery disease or stroke. Perhaps the most bothersome complication of these compounds is a diffuse arthralgia that may be disabling for older individuals; this complication is the major cause for treatment nonadherence.9 Before discontinuing the treatment, it is worthwhile to try a compound of a different class (i.e., a steroidal compound if the initial treatment was with a nonsteroidal compound and vice versa).
Unlike the SERMs, which combine antiestrogenic and estrogenic activities, fulvestrant (Faslodex) is a pure antiestrogen.12 It is administered by intramuscular injection every 4 weeks (after a loading regimen every 2 weeks 3 times). Like the SERMS and the AIs, fulvestrant may cause hot flashes; it can also cause vaginal dryness. The drug is active in patients whose disease has progressed while they were receiving SERMs or AIs. The long-term complications of this compound are poorly known because it has been used only in patients with metastatic disease and the treatment rarely lasted longer than 12 to 24 months. Estrogen in high doses has been the mainstay treatment of hormone-sensitive metastatic breast cancer before the SERMs became widely used and is still active in patients whose cancers progressed with other forms of hormonal treatment. However, high-dose estrogen causes an increased incidence of endometrial bleeding, deep vein thrombosis, fluid retention, and congestive heart failure.9 Ongoing studies are exploring the possibility of reversing acquired resistance to AIs by using estrogen in patients whose disease progressed after experiencing an initial response. Progestins are rarely used currently, as their activity is marginal after AIs. Likewise, androgens are rarely used because they cause virilization that is burdensome for most patients. Androgens may still have a role in palliating hormone-sensitive breast cancer in patients who are unsuitable for chemotherapy.
A detailed description of the chemotherapy agents used to treat breast cancer is beyond the scope of this chapter.3,13 They are the mainstay treatment of patients with hormone-unresponsive disease. In patients with metastases, most oncologists prefer to use single agents in sequence (to reduce the risk of complications) and reserve combination chemotherapy for patients with life-threatening metastases, such as lymphangitic lung metastases. All agents may cause neutropenia and thrombocytopenia, whose incidence and seriousness increases with age, and most, albeit not all, agents cause alopecia. The incidence of emetogenic complications used to be particularly high with doxorubicin and platinum derivatives but has been reduced dramatically in recent years thanks to new antinausea medications. The anthracyclines and anthracenediones may cause cardiomyopathy; the risk of this increases with patient age.14 The risk is reduced but not voided with pegylated liposomal doxorubicin or with the concomitant administration of doxorubicin and dexrazoxane. Peripheral neuropathy, which can be disabling for older individuals, is a common complication of taxanes, epothilones, vinblastine, and cisplatin.13 Another serious complication is mucositis, which is particularly common with doxorubicin, docetaxel, and fluorouracil.13 An unusual complication is the so-called hand-foot syndrome, manifested as pain and inflammation of the palms and the soles. This complication may be dose limiting for pegylated liposomal doxorubicin and capecitabine.15 Preferred single agents in the management of older individuals include capecitabine or weekly intravenous paclitaxel, nab-paclitaxel, or vinorelbine.16 Capecitabine is an oral prodrug that is activated in the liver and in the cancer itself. Older patients may prefer capecitabine because the dose can be titrated on a daily basis and does not require intravenous infusions.
Trastuzumab and pertuzumab are monoclonal antibodies directed against different domains of the HER2.17 In this situation, trastuzumab proved very effective in inducing durable complete remissions of breast cancer when combined with chemotherapy. Pertuzumab has limited activity as a single agent, but when used in combination with trastuzumab, it increases both the response rate and the response duration. The main toxicity of trastuzumab includes a reduction in the cardiac ejection fraction, as it may interfere with myocardial trophism and lead to a condition of “frozen myocardium.” This complication is more common in older individuals and is reversible in most cases. Recently, an immunoconjugate of trastuzumab with a very active cytotoxic agent, emtansine, was approved for clinical use in patients with breast cancer that progressed while they were receiving trastuzumab.18 In this case, trastuzumab is used as carrier of the drug to a specific target, the tumor, to enhance its effectiveness and to minimize its systemic complications. Lapatinib is an oral medication that inhibits the tyrosine kinase responsible for transducing the proliferative signals originating from an activated HER2.19 It is active after progression with trastuzumab, and it enhances the effectiveness of trastuzumab. In patients with a history of heart failure, it may be used in lieu of trastuzumab. Lapatinib penetrates the blood-brain barrier and may represent effective treatment and prevention of brain metastases. Everolimus is an inhibitor of mTOR (mammalian target of rapamycin), a threonine kinase that is a central step in signal transduction.20 In combination with the AIs, everolimus produces an approximately 20% response rate in patients whose disease has become resistant to hormonal therapy. This oral agent has significant toxicity that includes mucositis, bronchial irritation, and pneumonia, in addition to the financial toxicity. Bisphosphonates (pamidronate and zoledronate) and denosumab (a monoclonal antibody to the RANK ligand) delay the progression of bone metastasis and the occurrence of the so-called skeletal-related events.21 The bisphosphonates are administered intravenously and denosumab intramuscularly. A serious complication of all these compounds is osteonecrosis of the jaw, which includes failure to heal after oral surgery. Zoledronate has also been used to prevent osteoporosis in patients treated with AIs as adjuvant therapy and may further reduce the risk of systemic recurrence.10,11
The management of breast cancer varies with the stage of the disease.3 When the tumor is resectable, the treatment consists of partial or total mastectomy and SLN sampling. Full axillary dissection or radiation therapy to the axilla may be added in patients who test positive for cancer in the SLN. To ensure breast preservation, partial mastectomy should be followed by radiation therapy of the breast. Local treatment may be followed by systemic adjuvant therapy to reduce the risk of systemic recurrence, which is estimated according to several variables, including the patient’s life expectancy.3 The risk of recurrence increases with the size of the primary tumor, the number of lymph nodes involved, the tumor grade and proliferation rate, the absence of hormone receptors, and the presence of HER2 overexpression.
Adjuvant hormonal therapy is recommended in patients with hormone receptor–rich cancer. The most effective adjuvant hormonal therapy in postmenopausal women includes AIs.22 These may be used as a single treatment or may be followed by a SERM after 2 to 3 years. The concomitant administration of AIs and SERMs is not recommended, because the drugs may be antagonistic. SERMs alone may be used in patients who do not tolerate AIs. Adjuvant hormonal treatment should be administered for a minimum of 5 years. Recent studies indicate that a more prolonged treatment, up to a lifetime duration, may further reduce the risk of recurrence.22
Adjuvant chemotherapy in combination is indicated in patients with hormone receptor–negative tumors and in all patients with involvement of the axillary lymph nodes irrespective of the hormone receptor status.23 The analysis of the genome allows oncologists to identify a group of patients with negative nodes and hormone receptor–rich tumors that may benefit from adjuvant cytotoxic chemotherapy.24 In patients with HER2 overexpression, the addition of trastuzumab, for 1 year, to other systemic treatment has further reduced the risk of recurrence by more than 50%.
Sometimes the tumor is locally advanced and unresectable because of involvement of the chest wall or of the skin. In this situation, the primary treatment is systemic to reduce the size of the tumor and to make it amenable to local treatment, which may include both surgery and radiation.25
In the case of metastatic disease, the primary treatment is systemic.26 Chemotherapy is indicated in patients with hormone receptor–poor tumors and in those with hormone receptor–rich tumors that have progressed while receiving all forms of hormonal treatment. Irrespective of the hormone receptor status, combination chemotherapy is indicated in patients with life-threatening metastases, such as lymphangitic lung metastases. In patients with HER2 overexpression, the combination of trastuzumab, pertuzumab, and chemotherapy is the frontline treatment of choice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree