Breast Cancer
Benjamin T. Gielda
Katherine L. Griem
Adam Dickler
Introduction
Radiotherapy plays an integral role in the management of many patients with breast cancer whether they present with localized or disseminated disease. Treatment of breast cancer patients with radiotherapy requires knowledge of the anatomy of the breast and regional nodes as well as the treatment techniques available to encompass these volumes. This chapter discusses the management of clinically localized disease and describes the pertinent anatomy as well as the epidemiology and staging of breast cancer. We review the indications for irradiation of the intact breast and for irradiation in the postmastectomy setting, and we describe treatment techniques, volumes, and dose for these clinical situations.
Anatomy
Located within the superficial fascia of the anterior thoracic wall, the breast is composed of 15 to 20 segments of glandular tissue. Fibrous connective tissues connect the segments with adipose tissue abundantly interspersed between the segments. These segments compose the parenchyma and converge at the nipple in a radial fashion. Subcutaneous connective tissues surround the gland and extend as septa between the segments, providing structural support for the glandular elements. The deep layer of superficial fascia lies on the posterior surface of the breast adjacent to and at points fusing with the deep pectoral fascia of the chest wall. The retromammary bursa lies between the deep layer of superficial fascia and the deep pectoral fascia. It contains loose areolar tissue that allows for mobility of the breast over the chest wall. Fibrous bands of connective tissue attached between parenchyma extend from the deep pectoral fascia and attach to the superficial fascia (dermis of the skin). These ligaments, which are known as Cooper’s ligaments, insert perpendicular to the superficial fascial layers of the skin and permit mobility of the breast while also giving structural support.
The mature breast of the woman lies between the second rib superiorly and extends to the inframammary fold at the level of the sixth or seventh rib in the vertical axis. In the horizontal axis, it extends from the lateral edge of the sternum to the anterior or midaxillary line. The posterior surface rests on the deep pectoralis fascia, serratus anterior, external oblique abdominal muscles, and the upper rectus sheath. Breast tissue also projects into the axilla as the axillary tail of Spence, which may be very prominent. The upper half of the breast, the upper outer quadrant in particular, contains the greatest volume of glandular tissue.
The stroma and subcutaneous tissue of the breast contain fat, connective tissue, blood vessels, nerves, and lymphatics. The glands of the breast, derived from modified sweat glands of the epidermis, lie in the subcutaneous tissues. Each of the 15 to 20 segments of branched tubuloalveolar glands has collecting ducts draining them that are 2 mm in diameter and that terminate in subareolar lactiferous sinuses, which are 5 to 8 mm in diameter. Between 5 and 10 major collecting ducts open at the nipple.
Blood Supply
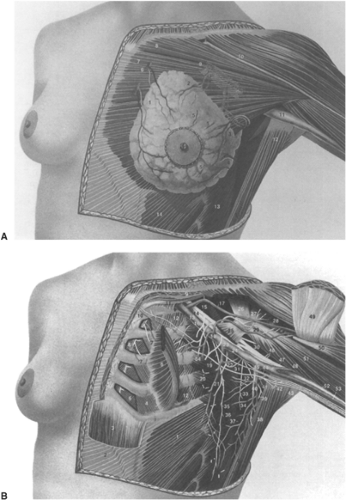
The breast receives its principal blood supply from the internal mammary (IM) and lateral thoracic arteries. The medial and central portions of the breast, ∼60%, are supplied by the anterior perforating branches of the IM artery. The remaining portion, the upper outer quadrant, is supplied by the lateral thoracic artery. Minor contributions to the arterial supply of the breast are supplied by subscapular and thoracodorsal arteries, the lateral branches of the third, fourth, and fifth intercostal arteries, and the pectoral branch of the thoracoacromial artery (Fig. 30.1A and B).
Lymphatic Drainage
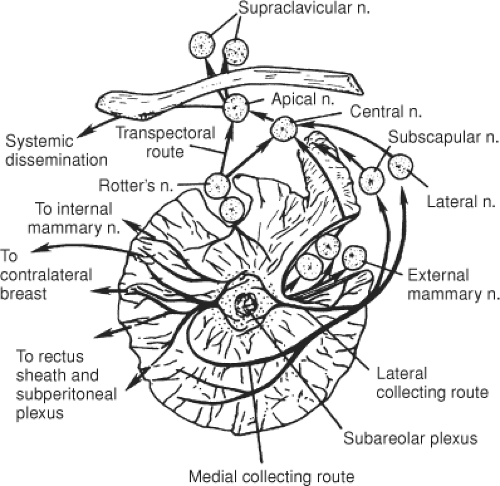
The most common sites of regional lymph node involvement in breast cancer are the axillary, IM, and supraclavicular regions. An understanding of the distribution and likelihood of involvement of the specific nodal groups is vital for treatment planning. More than 75% of lymph from the breast passes to the axillary nodes (Fig. 30.2), with the remainder flowing into parasternal lymphatics. The axillary nodes are thus the major site of regional metastases from breast carcinoma, and ∼40% of patients have histopathologic evidence of spread to the axillary nodes at presentation.
Physical examination of the axilla has both high false-positive and high false-negative rates in terms of determining axillary nodal involvement. Palpable axillary lymph nodes
are found to have evidence of metastatic disease in only 75% of patients. However, if enlarged axillary nodes are not palpated, metastatic disease is detected in ∼30% of the patients (1). This is important because the extent of axillary nodal involvement is the single most important prognostic factor in breast cancer. The 10-year survival of patients treated with radical mastectomy found to have negative axillary nodes is 65% to 80%, and with positive axillary nodes, 10-year survival is 25% to 48%. The prognosis is inversely related to the number of involved nodes (2).
are found to have evidence of metastatic disease in only 75% of patients. However, if enlarged axillary nodes are not palpated, metastatic disease is detected in ∼30% of the patients (1). This is important because the extent of axillary nodal involvement is the single most important prognostic factor in breast cancer. The 10-year survival of patients treated with radical mastectomy found to have negative axillary nodes is 65% to 80%, and with positive axillary nodes, 10-year survival is 25% to 48%. The prognosis is inversely related to the number of involved nodes (2).
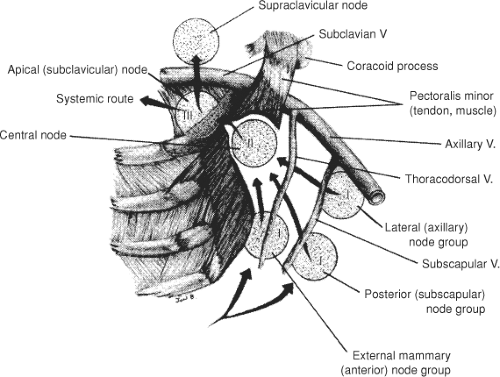
The anatomical arrangement of the axillary lymph nodes has several classifications. Pickren (3) has the most detailed studies of the pathologic anatomy of tumor spread. These groups include the following: (a) The axillary vein group (lateral group), which consists of four to six nodes medial or posterior to the vein (Fig. 30.3), receives most of the lymph drainage from the upper extremity. (b) The external mammary group, which contains five to six nodes along the lower border of the pectoralis minor, receives the majority of lymphatic drainage from the lateral breast. (c) The scapular group consists of five to seven nodes from the posterior wall of the axilla at the lateral border of the scapula. These nodes receive lymph from the lower posterior neck, posterior trunk, and posterior shoulder. (d) The central group consists of three or four large groups in the fat of the axilla posterior to the pectoralis minor muscle. This group not only receives lymph from the three preceding groups, but may also receive lymphatics directly from the breast. (e) The subclavicular group consists of 6 to 12 nodal groups posterior and superior to the upper border of the pectoralis minor. This group receives lymph from all groups of axillary nodes and unites with vessels from the subclavicular nodes to form the subclavian trunk. (f) The interpectoral (Rotter’s) group consists of one to four nodes interposed between the pectoralis major and inner muscles. Lymph from these nodes passes directly into the central and subclavicular groups.
Table 30.1 Internal Mammary (IM) Node Involvement in Relation to Site of Primary Tumor | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
An alternative and more commonly used method of determining pathological anatomy and metastatic progression is to divide the axillary nodal groups into three levels (Fig. 30.3). These levels are related to their position relative to the pectoralis minor muscle that inserts at the coracoid process. Level I lymph nodes lie lateral to the lateral border of the pectoralis minor muscle, level II nodes lie behind the pectoralis minor muscle, and level III nodes lie medial to the medial border of the pectoralis minor muscle.
The second major site of regional metastases for breast carcinoma is the IM lymph node chain. These nodes lie close to the IM vessels in extrapleural fat and are distributed in the intercostal spaces. The distribution of nodes in the intercostal space is as follows: first space, 97%; second space, 98%; third space, 82%; fourth space, 9%; fifth space, 12%; and sixth space, 62% (4). Initial studies by Handley (5) reported the results of IM node biopsies in 1,000 patients in 1975 and illustrated that IM node involvement is more common for inner-quadrant or central lesions than for outer-quadrant lesions (Table 30.1). Even in patients with inner or central lesions, the axilla is more commonly involved than the IM nodes: 42% versus 28%. Involvement of the IM nodes also correlates with tumor size. IM nodes are involved in 19% of patients with tumors smaller than 5 cm and 37% of patients with tumors larger than 5 cm. Injection studies with radiolabeled colloid demonstrate that drainage of lymph to the IM chain may be observed after injection of any quadrant of the breast.
The primary route of supraclavicular node involvement is through the axillary nodes. The significance of this nodal involvement was first shown by Halsted (6), who performed supraclavicular nodal dissection in 119 patients. Only 2 of the 44 women with positive supraclavicular nodes had no evidence of disease at 5 years. Thus, supraclavicular nodal involvement represents a late stage of axillary involvement and carries a grave prognosis. Supraclavicular lymph nodal involvement is classified as regional lymph node involvement rather than a site of metastatic disease in the most recent edition of American Joint Commission on Cancer (AJCC) Staging Handbook (7).
Local Involvement
The primary site of breast cancer is described by the quadrant of the breast that contains the carcinoma. In one series of 696 patients (8), 48% of the tumors were in the upper outer quadrant, 17% in the central region, 15% in the upper inner quadrant, 11% in the lower outer quadrant, and 6% in the lower inner quadrant. The frequency of a quadrant’s involvement is related to the proportion of breast tissue in that region. The relationship between the tumor location and prognosis was examined by the National Surgical Adjuvant Breast Project. Survival was found to be related to the pathological status of the lymph nodes but not to the primary tumor location (9) (Table 30.2).
The spread of cancer through the breast has been described by Haagensen (10). This spread occurs by direct infiltration into the breast parenchyma, along mammary ducts, and through the lymphatics. Direct infiltration occurs by projections having a characteristic stellate appearance. If the cancer is left untreated, involvement of skin and underlying fascia of the pectoralis muscle is
common. Intraductal components, which are frequently observed, may include more than one segment of the breast. It is not clear whether this is spread of the primary cancer or a field cancerization that results in transformation of the ductal lining. Because of lymphatic spread vertically to the plexus of the pectoralis fascia and spread to the central subareolar region, cancer beyond the palpable mass may be present in the breast.
common. Intraductal components, which are frequently observed, may include more than one segment of the breast. It is not clear whether this is spread of the primary cancer or a field cancerization that results in transformation of the ductal lining. Because of lymphatic spread vertically to the plexus of the pectoralis fascia and spread to the central subareolar region, cancer beyond the palpable mass may be present in the breast.
Table 30.2 Five-Year Recurrence Rate According to Tumor Location and Nodal Status | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Epidemiology
It is estimated that 192,730 new cases of invasive breast cancer and 62,280 cases of in situ disease will be diagnosed in the United States during 2009. Breast cancer will account for about 27% of all new cancers in women. Approximately 40,170 women will die of breast cancer, making breast cancer second only to lung cancer with respect to female cancer mortality (11). The cumulative lifetime estimated risk of developing breast cancer is one in eight for U.S. women (11). The factors that increase the risk of developing breast cancer include family history, reproductive history, exposure to radiation and hormones, age over 50, nulliparity, and first child after age 30. Even so, ∼70% of women who develop breast cancer will do so without identifiable risk factors. Age-adjusted mortality rates in the United States have declined by ∼3% per year since 1989, possibly due to improved screening and treatment (12). A similar trend has been observed in the United Kingdom where the overall breast cancer mortality rate has fallen 36% since 1989 (13).
Many studies have found a link between the incidence of breast cancer and the age at menarche, menopause, and first pregnancy. An early age at menarche has been associated with an increased risk of developing premenopausal and postmenopausal breast cancer. Later onset of menarche, which is associated with a delay in the beginning of regular cycles, suggests a 20% decrease in breast cancer risk for every year delayed (14). Recent evidence demonstrates that early menarche, while altering the incidence of all major histological subtypes of breast cancer, may increase the risk of lobular carcinoma the most (15).
Another risk factor for breast cancer is age at menopause. The risk of breast cancer increases by about 3% per each year in delay of age at menopause, and this risk extends to all histologic subtypes (15,16). This information led to the general acceptance that it is the total duration of estrogen exposure and its resultant effect on cell division that affects the risk of breast cancer.
Breast cancer risk is also affected by other factors that influence hormonal exposure, including parity and age at birth of the first child. Nulliparous women are at greater risk for developing breast cancer than parous women. This risk is modulated by age at first full-term pregnancy, as women who have their first pregnancy after the age of 30 have a twofold to fivefold higher risk of developing breast cancer than women whose first pregnancy is before age 20. An abortion of any kind does not have the protective effect of a full-term pregnancy and may even increase the risk of cancer (17). During an incomplete pregnancy, the breast is exposed only to high levels of estrogen and is thus unable to undergo the complete differentiation that results from a term pregnancy.
The association between oral contraceptive use and breast cancer is complex. In the United States, the trend has been that the duration of contraceptive use has increased, and age at first use of oral contraceptive pills has decreased. Most studies have shown no significant increase in the breast cancer risk with long durations of use (16,18). Several studies have shown a transiently increased risk of breast cancer with current or recent use (<10 years since last use) in women younger than age 45 (19,20).
Multiple sources suggest that postmenopausal hormone replacement may be associated with an increased risk of breast cancer, with the degree of risk related to the duration of use (21,22,23). To date, the effect of this increased incidence on mortality has not been determined, but in general, cancers developing in the setting of estrogen replacement have a relatively favorable prognosis (24). There is little information regarding the use of low-dose therapy over a short time. Thus, the risk versus benefits should be considered on an individual basis prior to the initiation of therapy.
Women who have a strong family history of breast cancer demonstrate an increased risk of breast cancer. The risk is increased 1.5 to 3 times if a first-degree relative has breast cancer; the risk is greater if the affected relative developed their breast cancer prior to menopause (25). Only 5% to 10% of breast cancer patients have a family history consistent with hereditary carcinoma. Hereditary breast carcinoma has been linked to the BRCA 1 gene located on the long arm of chromosome 17 (17q21). Carriers of BRCA 1 mutation have 36% to 56% lifetime risk of developing a breast carcinoma and are also at increased risk for developing ovarian cancer (26). BRCA 1 accounts for approximately 40% to 50% of hereditary breast cancers. An additional gene, BRCA 2 accounts for a portion but not all of the additional cases of hereditary breast cancer (27).
There is clear evidence that exposure to ionizing radiation of any source, especially during and after puberty, increases the risk of breast cancer, and that the degree of risk is dose dependent (28,29,30,31). Land et al. (32) reviewed studies of exposed populations and concluded that the risk of radiation-induced breast cancer increased linearly according to dose and the age at exposure. There is a long latent period between exposure and the development of breast carcinoma; therefore exposure after age 40 causes only a minimal increase in risk. It should be stressed that the benefit to women from screening mammography outweighs any increased risk of breast cancer.
The currently available data do not support a significant causal effect between manmade chemical exposure and breast cancer. In addition, active and passive smoking
have not been shown to increase the overall rates of breast cancer (33). A large review of >100 studies did not show a significant association between breast cancer and specific occupations (34).
have not been shown to increase the overall rates of breast cancer (33). A large review of >100 studies did not show a significant association between breast cancer and specific occupations (34).
Staging
The current staging system in widespread use is a joint system developed by the American Joint Committee on Cancer (AJCC) and Union for International Centre Cancer (UICC). This staging system uses the TNM system of staging and information obtained before surgery or postsurgical pathologic results. The seventh edition of the staging system is presented in Table 30.3 (7).
Treatment
Postmastectomy Radiotherapy
Randomized studies, from British Columbia and the Danish Breast Cancer Cooperative Group, have shown a benefit in high risk patients from the addition of radiotherapy to modified radical mastectomy combined with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy (35,36). These studies included patients who were node positive and premenopausal. The addition of radiotherapy to chemotherapy improved local recurrence, disease free survival, and overall survival. The benefit of radiation in combination with Tamoxifen has also been shown in high risk postmenopausal patients (37). The Early Breast Cancer Trialists’ Collaborative Group performed a meta-analysis analyzing 8,500 women with node positive breast cancer receiving mastectomy and axillary clearance. Radiotherapy was associated with an absolute improvement of 17% in locoregional control at 5 years, that subsequently translated into an absolute overall survival benefit of 4.4% at 15 years (38).
Indications
The rationale for postmastectomy radiotherapy is to prevent recurrence of cancer in the chest wall, skin, mastectomy scar, and the regional nodes, including the axillary, supraclavicular, and IM nodes. Available evidence suggests that prevention of local failure has a positive impact on survival (38). Prevention of recurrence is an important end point. Local recurrence is often symptomatic; these recurrences can ulcerate and be a source of bleeding as well as pain and infection. Furthermore, regional recurrence, particularly in the axilla and supraclavicular regions, can be symptomatic and cause significant pain from brachial plexus involvement. Local and regional recurrences are difficult to control once they occur. In one series (39), two-thirds of patients who failed locally after mastectomy died with uncontrolled locally recurrent breast cancer. Finally, local recurrences may have the ability to seed distant recurrence; prevention may decrease the likelihood of the development of metastatic disease (40).
Careful selection of patients for postmastectomy irradiation is critical, given the potential morbidity of treatment. Following publication of the British Columbia and Danish randomized trials, consensus criteria were formulated recommending postmastectomy radiotherapy for patients with four or more involved lymph nodes and tumors larger than 5 cm (41,42). Other frequently used indications drawn from prospective and retrospective experiences include involved nodal ratio >0.20, invasion of pectoral fascia, lymph-vascular space invasion, positive margins, involvement of the skin or nipple, gross multicentric disease, and gross extracapsular lymph node spread (35,37,43,44,45) Further analysis of the Danish Trials demonstrated a benefit in patients with one to three positive nodes as well, but radiotherapy in this subset remains controversial (46).
The use of neoadjuvant chemotherapy has increased in recent years. Indications for postmastectomy radiotherapy in patients treated with neoadjuvant chemotherapy are less clear secondary to the lack of randomized prospective data. Recent consensus guidelines based on large retrospective experiences recommend postmastectomy radiotherapy for patients presenting with stage III disease or with lymph node involvement at the time of resection (47).
Simulation Technique
Treatment of the chest wall and regional nodes is traditionally accomplished by matching a supraclavicular field to tangents treating the chest wall. Careful treatment planning is necessary to avoid excessive irradiation of normal tissue while encompassing the areas at risk without overlap. Several methods to achieve an ideal match between the caudal edge of an anterior supraclavicular port and the cephalad edge of two opposed tangential fields have been described. The supraclavicular port is usually treated with a half-beam technique to achieve a nondivergent edge, and some form of mounted or hanging block, couch rotation, and/or collimator angulation is used on the tangential fields to achieve a match (48,49,50,51). A simulation technique for comprehensive postmastectomy irradiation using a conventional simulator, requiring only an asymmetric collimator on the treatment machine and breast tilt board, has been previously described in detail (52,53). Below is presented the CT adaptation of the three field technique used at Rush University Medical Center.
Three-Field Single Isocenter
The patient is placed supine with both arms above the head. Raising both arms is desirable should it become
necessary to use intensity-modulated radiation therapy (IMRT). We attempt to make the chest as parallel to the simulation table as possible, using the breast board as necessary. The clinical boundaries of the intended treatment field, mastectomy scar, and drain sites are marked with radioopaque wire. The medial border splits the sternum and connects the center of the xiphoid process with the center of the thoracic inlet. The lateral border is defined by the mid-axillary line. The superior border is the inferior edge of the clavicular head. The inferior border is placed 1 to 2 cm below the location of the inframammary fold. The contralateral breast, if present, is typically used to aid in placing the inferior border. An IV is placed in the contralateral upper extremity if there is no contraindication to IV contrast.
necessary to use intensity-modulated radiation therapy (IMRT). We attempt to make the chest as parallel to the simulation table as possible, using the breast board as necessary. The clinical boundaries of the intended treatment field, mastectomy scar, and drain sites are marked with radioopaque wire. The medial border splits the sternum and connects the center of the xiphoid process with the center of the thoracic inlet. The lateral border is defined by the mid-axillary line. The superior border is the inferior edge of the clavicular head. The inferior border is placed 1 to 2 cm below the location of the inframammary fold. The contralateral breast, if present, is typically used to aid in placing the inferior border. An IV is placed in the contralateral upper extremity if there is no contraindication to IV contrast.
Table 30.3 AJCC Staging for Breast Cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Scout images are obtained to verify patient setup. Axial 3-mm CT slices are acquired from the angle of the mandible through the entire thorax, taking care to include the aforementioned inferior border plus at least 4 cm. IV contrast is infused to improve nodal basin delineation. A four-dimensional (4D) simulation is performed to assess motion of the chest wall. A more thorough discussion of respiratory motion follows later in this chapter. Breath-holding techniques or gating, also discussed later, may be used if available, particularly in the setting of left-sided cancers.
Following acquisition, the scan is manipulated using the simulator software. Contours are placed on the CT slices containing the superior and inferior wires respectively. The CT slice midway between the superior and inferior wires is selected. A line is drawn between the medial and lateral wires on this slice. The distance of the line is measured and a point is placed at the midpoint of the line. This point represents the psuedoisocenter. Opposed tangents are created with a shared central axis that intersects the psuedoisocenter, the medial wire, and the lateral wire. The superior and inferior jaws are set to their respective clinically defined wires. The x, y, and z coordinates of the pseudoisocenter are recorded. The CT scan is then advanced to the slice containing the superior wire. This slice defines the match plane. The true isocenter is placed on this slice containing the superior wire, with the same x and y coordinates as the psuedoisocenter. The tangent fields are tagged to the true isocenter; the superior jaw is set to zero, and the inferior jaw is opened to the inferior wire. The tangents are now a quarter beam encompassing the intended chest wall, with a nondivergent superior edge. A supraclavicular field is created and tagged to the true isocenter. The supraclavicular inferior jaw is set to zero, the superior jaw is set to the level of the cricoid cartilage, the medial jaw is set to the vertebral bodies, and the lateral jaw is set to cover either the level III axillary nodes and supraclavicular nodes or the whole axilla, depending on the clinical situation. The single isocenter is marked on the patient, and the images are transferred to the treatment planning system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree