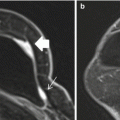
Fig. 12.1
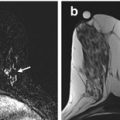
An axial fat saturated contrast enhanced subtraction image showing a focal area of non mass-like enhancement in the outer right breast (arrow). This was not demonstrated mammographically or on ultrasound and was considered indeterminate. An MRI guided biopsy was recommended
Contraindications for breast MRI biopsy are the same as those for a diagnostic MRI (pacemaker, other implantable devices etc.), contrast medium injections (allergy, severe renal impairment) and biopsies (poor coagulation, allergy to local anesthesia) [18, 19]. These contraindications may be relative and careful consultation with clinical colleagues such as cardiologists and hematologists may facilitate the biopsy procedure depending on each individual case. Radiofrequency excisional biopsy devices (Intact®) cannot be used because of interference with the electromagnetic wave.
MRI-guided biopsies should only be carried out in experienced breast centers where these are preformed regularly [20–22]. The team must have suitable experience in performing both breast MRI and vacuum-assisted breast biopsy, although the exact training requirements in MRI-guided vacuum-assisted biopsies varies enormously internationally. In some countries where access to MRI is more limited, the initial training involves only a few procedures, but 15 procedures are required according to the European guidelines [20].
12.2 Technical Aspects
Most MRI scanners currently used in clinical practice have a field strength of either 1.5 T or 3 T. In the latter system the sensitivity of detecting the cancer is greater for the same specificity [23], although artefacts are generally increased. Susceptibility artifact is more than double in size at 3 T vs 1.5 T [24].
Open MRI scanners in theory provide easier access to the breast and real time monitoring of insertion of the cannula. However, to date these scanners utilize a low field (0.2–0.5 T), which is not of sufficient quality imaging for breast imaging [25].
The coils used for biopsy should if possible be the same as those used for diagnosis in order to reproduce the diagnostic scan (and hence lesion requiring biopsy) as close as is possible. It must be possible to access the breast to take the samples, which assumes that the coil is open. Current dual breast coils allow either external, internal or even superior access although the lateral approach is preferred as this is technically the most straightforward as shall be discussed (Fig. 12.2).


Fig. 12.2
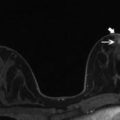
An image showing a breast biopsy compatible MRI coil
As an alternative to biopsy coils, perforated plate systems can be used together with flexible ring coils placed around the breast. Perforated plate systems are sometimes advantageous for reaching findings close to the thoracic wall. Compared to multi-channel breast biopsy coils, however, a ring coil is associated with a reduced signal-to-noise ratio and thus inferior image quality. This is true particularly for findings far from the coil (for example near the nipple).
Internal access is limited for deep (medial) lesions and this is technically more challenging. However historically where the whole breast would be traversed by the biopsy system, the contralateral breast can now be positioned on a board and the radiologist works from beneath in a tunnel. In principle, the shortest possible access should be selected and newer generation coils allow for medial and lateral access for biopsy. The medial access may be more difficult due to the longer distance in conjunction with the reduced light and operating space beneath the patient. As a general principal post-biopsy, a clip insertion is recommended to ensure the ability for localization through subsequent ultrasound or mammographic guided wire marking of the clip.
Regardless of targeting method, an opaque landmark such as a vitamin E capsule is attached to the compression plate. The end of this is positioned in contact with the breast and used as the landmark for the three spatial planes and to allow subsequent targeting. This appears as a focal area of hyperintensity on the unenhanced T1 weighted views.
The MRI scanner itself will likely have a targeting software package or this can be obtained separately depending on the manufacturer. These are particularly useful for posterior contrast enhancement. Computer aided detection software (CAD) purchased usually as a stand alone software package can facilitate better lesion delineation particularly in relation to the subtraction imaging. The biopsy system used is then computed with calculation of the necessary depth taking account of the materials and thickness of the grid.
The principle used is that the same image is taken on four occasions: before biopsy (target identification), after positioning a guide (checking correct position of the biopsy system), after taking the biopsy (confirming that the biopsy cavity is consistent with the target) and after positioning the clip (checking the correct position of the marker).
Initial and then dynamic images are preferably taken in high- resolution T1 weighted sequences. This could be a 2D exam but is preferably a 3D echo gradient with fat saturation [26]. It is ideally the highest possible spatial resolution at a temporal resolution of 60–120 s per series, with either transverse or sagittal slice orientation. The acquisition may be taken through axial sections although resolution is often better in sagittal sections [27]. For reliable lesion imaging, subtraction series of every contrast enhanced series should be acquired. Rapid T1 W spin echo (TSE) images are preferable in order to reduce artifact from the needles [27].
The maximum intravenous contrast dose (0.2 ml/kg) or a half dose is injected depending on whether or not a repeat end of procedure injection is planned. This is performed at an injection rate of 2–3 ml/s and the contrast agent is then washed out with a subsequent bolus injection of 20 mls of physiological saline solution (0.9 % NaCl).
The Mammotome® (Devicor Inc., Cincinnati, USA) was the earliest available vacuum biopsy system and was used for MRI-assisted biopsy in the late 1990s. However currently there are several manufacturers that now produce equipment for MRI-guided VAB of the breast. In Europe the EnCorTM (Senorx or Enspire, Bard GmbH, Karlsruhe, Germany) and the ATEC® (Hologic Inc., Bedford, USA) are now widely popular and have superseded the less automated Vacora® (Bard GmbH, Karlsruhe, Germany) system.
MRI-guided VAB was initially performed using an 11-gauge needle, but as with mammogram guided VAB, MRI-guided vacuum-assisted biopsy have trended toward larger needle gauges (up to 7G). These allow the collection of the same tissue volume with fewer samples in a shorter examination time. There are no specific guidelines defining the number of samples for MRI-VAB, but a European consensus paper on the use of MRI-VAB recommends taking at least 24 11G samples or an equivalent tissue volume if larger needle gauges are used [21]. However, the recommended sample here is based on very limited evidence. The number of samples reported in the literature ranges from 2 to 75 with a median of 12 [28–33].
Most VAB devices have a cable connection to the vacuum source located outside the MRI examination room. Non-magnetic materials should be used in preference to ferromagnetic materials (needles, biopsy guns, etc.) in order to minimize the chances of an accident from magnetic attraction. As these various guns are non-magnetic (Vacora® less than the others), they are not attracted by the magnet although interference with their operation does occur if they come too close to the magnet.
The Vacora® is a battery-operated system and thus a true handheld system. The disadvantage of this system is that the device has to be removed from the breast after each sample is taken. This causes more difficulty from blood [30] and air and it is essential to use a support for the gun in order to reduce the risk of displacing the cannula. The vacuum aspirate is reported to be less powerful and the sampling process slower (69 min vs 39 min). Automated coaxial systems are reported to be able to biopsy smaller lesions (10 mm vs 19 mm) in a shorter exam time [34]. While the automated devices mentioned also take individual samples, the biopsy system remains in the breast during the entire intervention. The samples are then automatically transported to a chamber in the handle, where they can later be removed. The ATEC® and EnCorTM provide the advantage of the automated removal of multiple samples in immediate succession. The ATEC® additionally provides the option of rinsing the biopsy cavity with saline.
12.3 The Procedure
Efficiency and speed are of particular importance during this type of biopsy procedure. Because of the transient nature of contrast enhancement on MRI. there is a narrow window of time in which to perform the procedure and verify needle placement. Although variable to some extent, a 15–20 min time frame is expected. The more prolonged the procedure becomes, the more likely the contrast will wash out and also the more likely the patient is to move, resulting in motion artifact and potentially leading to incorrect targeting.
Patient positioning may vary slightly depending on institutional practice. The patient may be positioned on her side with her head turned to the opposite side and her arm above her head. Alternatively, the patient’s head may be placed on a head rest or positional device so that the patient is looking straight down. A venous line with long connection tubing is in place. The breast is wedged in the surface coil and the guiding system is set up from the beginning. The skin marker is positioned in contact with the skin as close as possible to the projection of the lesion if no CAD system is being used, or further away in order to avoid obstruction of it if one is being used. Vitamin E capsules are often used as fiducial markers and are taped over the expected site of the lesion.
Modest compression is used to avoid masking the enhancement [35] and to reduce the accordion effect (decompression of the breast may cause displacement of a clip or coil). Accessibility of the presumed site of the lesion is then checked and positioned in the effective grid compression area (Fig. 12.3).


Fig. 12.3
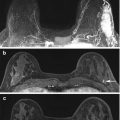
An image showing a patient within the breast biopsy coil and demonstrating the grid localisation system
The patient is brought into the magnet and an initial contrast enhanced image is taken to find the lesion and locate it against the opaque landmark (this usually appears as a T1 weighted hyperintensity on the unenhanced image) (Fig. 12.4). Distances are measured manually or by software in the 3 spatial planes between this reference point (“zero”) and the lesion.


Fig. 12.4
A pre biopsy fat saturated contrast enhanced sagittal image showing the grid system over the skin allowing appropriate skin marking for needle entry point
After sterile preparation the local anesthesia is administered. In the absence of a contraindication, this usually consists of a large volume of lidocaine with epinephrine (lidocaine HCL 1 % and epinephrine 1:100,000). 20–40 cm3 is commonly infiltrated in split doses, with 10–20 cm3 administered before insertion of the biopsy device, and 10–20 cm3 is administered by the device just prior to and during sampling. Epinephrine may sometimes minimise parenchymal hematoma formation, which amongst other things could potentially obscure the biopsy site. Initial subcutaneous anesthesia, however, is ideally obtained by using a small volume of lidocaine only with epinephrine not administered to the skin. It is of particular importance to ensure that no air bubbles are present within the syringe at the time of administration as even small air bubbles can cause significant artifact on the MRI.
Following the anesthetic, a skin incision is made. Depth is then adjusted by adding 20 mm for Senorx®, 10 mm for Vacora®, but nothing for Mammotome®. Once in place the metal sheath is replaced with a silicone sheath or with the position marker. The patient is returned inside the magnet and a rapid image is then taken to check the correct position of the introducer (Figs. 12.5, 12.6, 12.7, and 12.8).
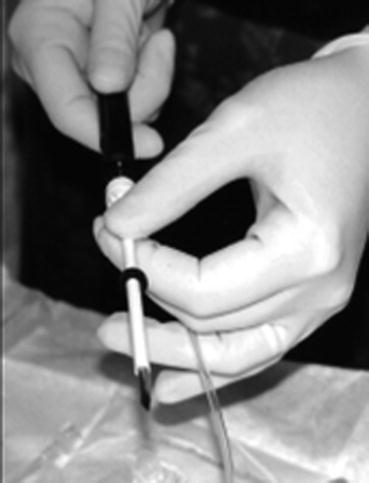
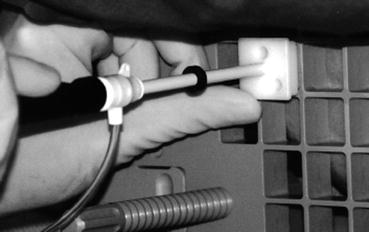
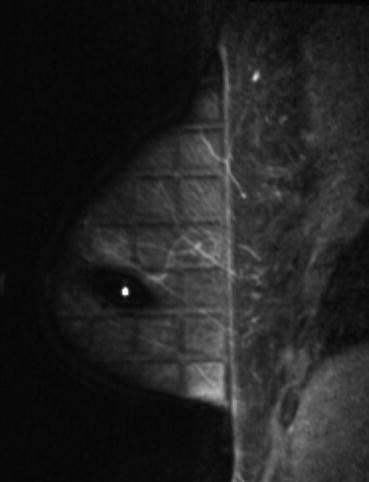
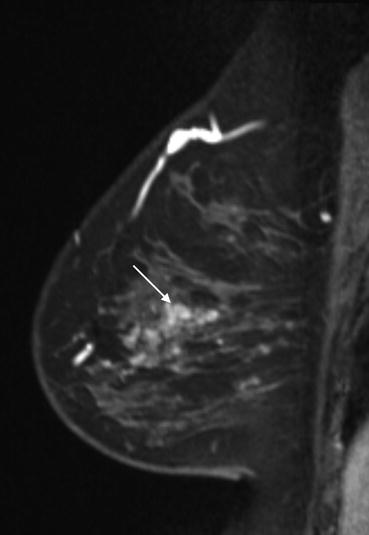

Fig. 12.5
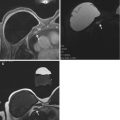
An image showing the needle introducer being assembled prior to MRI guided biopsy

Fig. 12.6
An image showing a patient within the breast biopsy coil and demonstrating the introducer being passed through the grid localisation system

Fig. 12.7
A pre biopsy fat saturated contrast enhanced sagittal image showing the grid system over the skin with the biopsy needle passing through the image in position for biopsy

Fig. 12.8
A prelocalisation fat saturated contrast enhanced sagittal image demonstrates the lesion persists (arrow) and therefore a MRI biopsy was performed
The introducer is replaced by the cannula and then a series of samples are taken. The number of samples depends on the size of the lesion and quality of targeting. The ability to sample in a designated direction is a major advantage to performing this test with a vacuum biopsy needle. Prior to sampling it may be obvious that the lesion is slightly eccentrically site in relation to the needle tip. In this situation, the biopsy window can be targeted towards the lesion rather than just sweeping a full 360 degree circle. Early rounds of sampling usually produce the highest yield and the more samples that are obtained, the more likely it is that there will be hematoma formation in the target area. The result of this is that the biopsy device becomes more distant from the target lesion and there are thus diminishing returns of later and continued sampling in this scenario. For MRI guided biopsies, it is important to remember that the clock face is relative to the grid and not to the breast or to the patient. The aperture of the vacuum needle needs to be adjusted to reflect this. The samples are then placed in formalin and sent to pathology. The specimens are fixed and then sectioned and interpreted by an experienced breast histopathologist.
A marker clip is routinely positioned as this may be only landmark, which could be used to guide any subsequent surgery if required [29, 36–39]. It is ideally placed through the cannula prior to its removal or alternatively following the check image, through the introducer. The patient is repositioned in the tunnel for a final sequence in order to determine whether the contrast uptake dissipated although it is often sufficient to check that the biopsy area is correctly centered on the lesion (by comparing with the pre-biopsy image) and that the clip has been deployed. This sequence is carried out with or without contrast enhancement and may facilitate further sampling or lesion retargeting (Fig. 12.9).
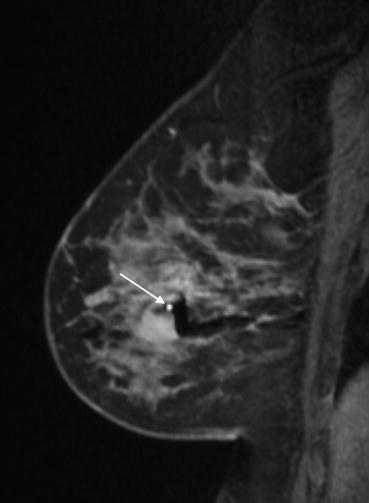

Fig. 12.9
A mid biopsy contrast enhanced sagittal image showing the needle within the target lesion (arrow). Biopsy related hematoma is demonstrated as signal dropout (black areas around the needle)
At the termination of the procedure, the patient is removed from the tunnel, placed flat on her back and manual compression to the breast biopsy site is applied followed by a compressive dressing. Monitoring following the procedure should be as per local protocols for a vacuum biopsy and be dependent on various patient factors as well as the degree of hematoma that has formed.
Signal void from the marker clip may be indistinguishable from signal void from air introduced during the procedure and so in order to ensure that the marker has deployed correctly, a post biopsy mammogram is usually recommended. A craniocaudal and mediolateral mammogram would typically be obtained. The position of the marker clip on the mammogram should be compared with the expected site of the lesion based on the diagnostic MRI examination. Any marker displacement needs to be clearly noted as a future wire localization may be required dependent on the histopathology from the biopsy.
Multiple lesions can be attempted at a single appointment although this may be challenging even for the most tolerant patient. As with any biopsy procedure, the most suspicious lesion should undergo intervention first, in case the later sites are not visualized or the patient is unable to continue. When dealing with multiple lesions in the same breast, the most favourable scenario is if the lesions can be positioned beneath the grid surface simultaneously so that access to both sites can be obtained without the need to reposition. In succession, both lesions are localised, anesthetized and then introducer stylets inserted prior to biopsies. If multiple lesions within a single breast cannot be positioned at the same time (or indeed there are bilateral lesions), then the more suspicious lesion is sampled first and sampling at this site completed (including marker deployment). If washout does occur because of the time elapsed between the gadolinium injection and biopsy at the second site, then landmarks may be adequate to guide the procedure.
12.4 Pitfalls and Limitations
Unfortunately despite the latest MRI technical developments there is a procedural failure rate. This rather varies in the literature as to the frequency but may be up to 25 % [40–44]. This will occur most commonly due to either non visualisation of the target lesion or an inaccessible target area. The target may not be seen because it has disappeared due to excessive compression. In this situation a further image could be performed with less breast compression. Alternatively the initial MRI may have been performed at the wrong time of the menstrual cycle and as such the target is no longer identifiable. This masking effect is more common in smaller sized targets (<5 mm), and where background enhancement may also obscure the area [40, 45]. If indeed the target demonstrates a clear decrease in size at the time of the procedure compared to the original MRI scan then that is an indication not to perform the biopsy.
Motion artefacts can also cause false positive findings on MRI in particular on subtraction images of the T1-weighted contrast enhanced series, where they result in hyperintense findings that could be interpreted as lesions of increased contrast enhancement. To avoid these false positives, the unsubtracted series should also be evaluated [17, 28]. Overly forceful breast compression may result in reduced contrast enhancement. If there is suspicion of this, then a repeat MRI with less breast compression would be recommended. Alternatively a delayed MRI sequence may sometimes be valuable in demonstrating the target even if the early subtraction views do not [33, 46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree