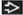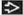(T) Primary Tumor | Adapted from 7th edition AJCC Staging Forms. | ||
TNM | Definitions | ||
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
Tis | Carcinoma in situ | ||
Tis (DCIS) | Ductal carcinoma in situ | ||
Tis (LCIS) | Lobular carcinoma in situ | ||
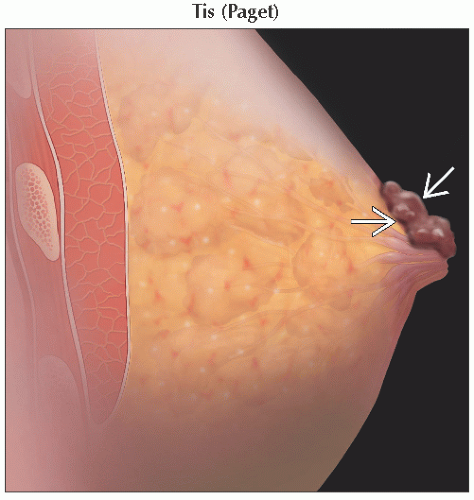
Tis (Paget) | Paget disease of the nipple not associated with invasive carcinoma &/or carcinoma in situ (DCIS &/or LCIS) in the underlying breast parenchyma; carcinomas in the breast parenchyma associated with Paget disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget disease should still be noted | ||
T1 | Tumor ≤ 20 mm in greatest dimension | ||
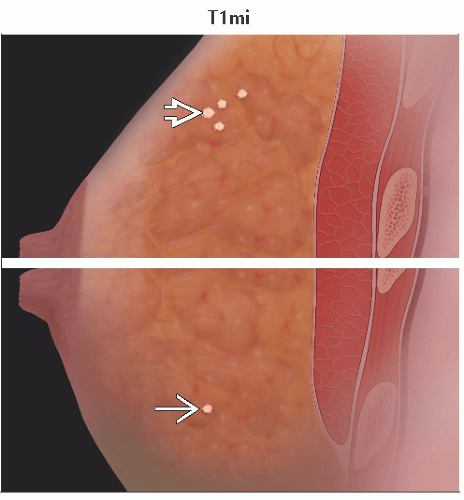
T1mi | Tumor ≤ 1 mm in greatest dimension | ||
T1a | Tumor > 1 mm but ≤ 5 mm in greatest dimension | ||
T1b | Tumor > 5 mm but ≤ 10 mm in greatest dimension | ||
T1c | Tumor > 10 mm but ≤ 20 mm in greatest dimension | ||
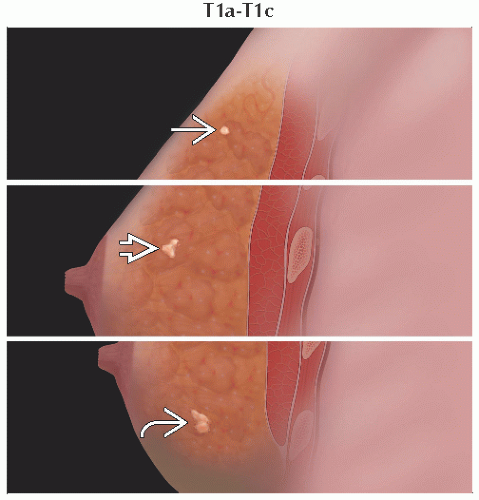
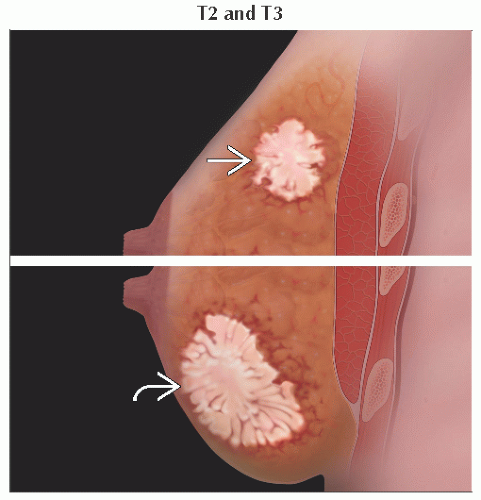
T2 | Tumor > 20 mm but ≤ 50 mm in greatest dimension | ||
T3 | Tumor > 50 mm in greatest dimension | ||
T4 | Tumor of any size with direct extension to the chest wall &/or to the skin (ulceration or skin nodules); invasion of the dermis alone does not qualify as T4 | ||
T4a | Extension to the chest wall, not including only pectoralis muscle adherence/invasion | ||
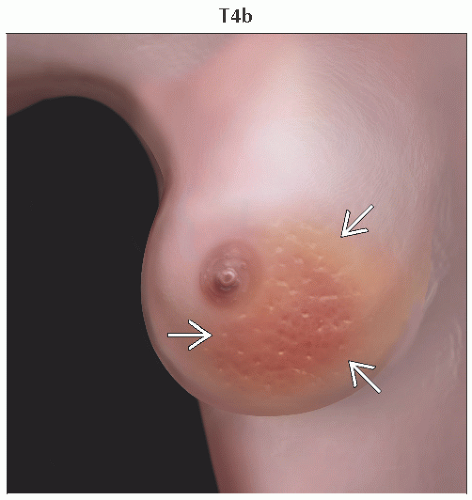
T4b | Ulceration &/or ipsilateral satellite nodules &/or edema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma | ||
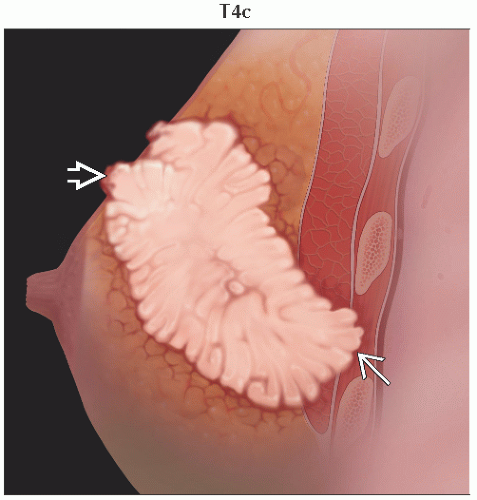
T4c | Both T4a and T4b | ||
T4d | Inflammatory carcinoma | ||
The T classification of the primary tumor is the same regardless of whether it is based on clinical or pathologic criteria, or both. Size should be measured to the nearest millimeter. If the tumor size is slightly less than or greater than a cutoff for a given T classification, it is recommended that the size be rounded to the millimeter reading that is closest to the cutoff. For example, a reported size of 1.1 mm is reported as 1 mm or a size of 2.01 cm is reported as 2.0 cm. Designation should be made with the subscript “c” or “p” modifier to indicate whether the T classification was determined by clinical (physical examination or radiologic) or pathologic measurements, respectively. In general, pathologic determination should take precedence over clinical determination of T size. | |||
(N) Regional Lymph Nodes, Clinical Classification | Adapted from 7th edition AJCC Staging Forms. | ||
TNM | Definitions | ||
NX | Regional lymph nodes cannot be assessed (e.g., previously removed) | ||
N0 | No regional lymph node metastases | ||
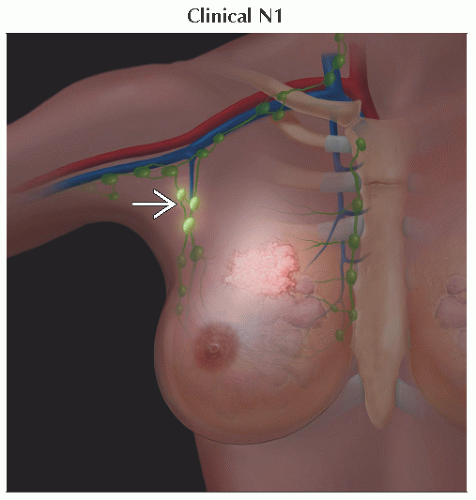
N1 | Metastases to movable ipsilateral level I, II axillary lymph node(s) | ||
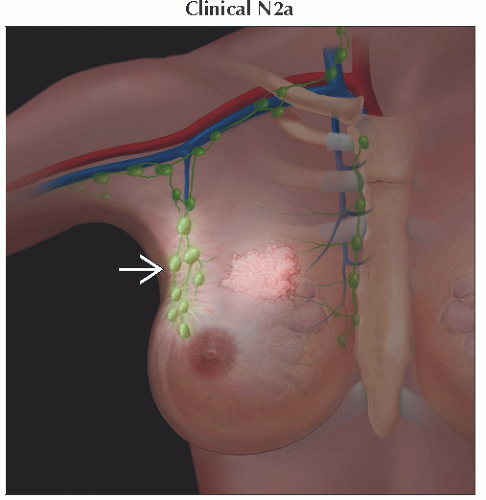
N2 | Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted; or in clinically detected* ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases | ||
N2a | Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures | ||
N2b | Metastases only in clinically detected* ipsilateral internal mammary nodes and in the absence of clinically evident level I, II axillary lymph node metastases | ||
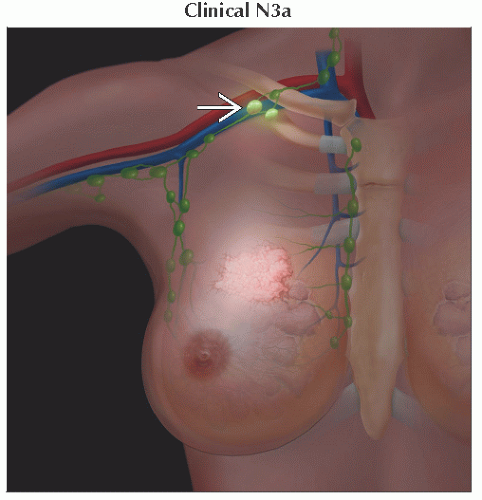
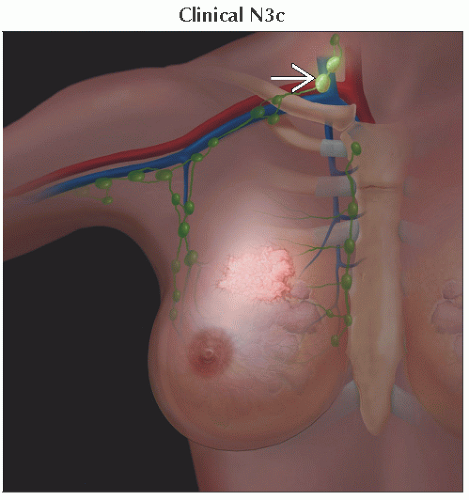
N3 | Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s) with or without level I, II axillary lymph node involvement; or in clinically detected1 ipsilateral internal mammary lymph node(s) with clinically evident level I, II axillary lymph node metastases; or metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement | ||
N3a | Metastases in ipsilateral infraclavicular lymph node(s) | ||
N3b | Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) | ||
N3c | Metastases in ipsilateral supraclavicular lymph node(s) | ||
* “Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination and having characteristics highly suspicious for malignancy or a presumed pathologic macrometastasis based on fine needle aspiration biopsy with cytologic examination. Confirmation of clinically detected metastatic disease by fine need aspiration without excision biopsy is designated with an (f) suffix, for example, cN3a(f). Excisional biopsy of a lymph node or biopsy of a sentinel node, in the absence of assignment of a pT, is classified as a clinical N, for example, cN1. Information regarding the confirmation of the nodal status will be designated in site-specific factors as clinical, fine needle aspiration, core biopsy, or sentinel lymph node biopsy. Pathologic classification (pN) is used for excision or sentinel lymph node biopsy only in conjunction with a pathologic T assignment. | |||
(pN) Pathologic Lymph Node Classification1 | Adapted from 7th edition AJCC Staging Forms. | ||
TNM | Definitions | ||
pNX | Regional lymph nodes cannot be assessed (e.g., previously removed, or not removed for pathologic study) | ||
pN0 | No regional lymph node metastasis identified histologically | ||
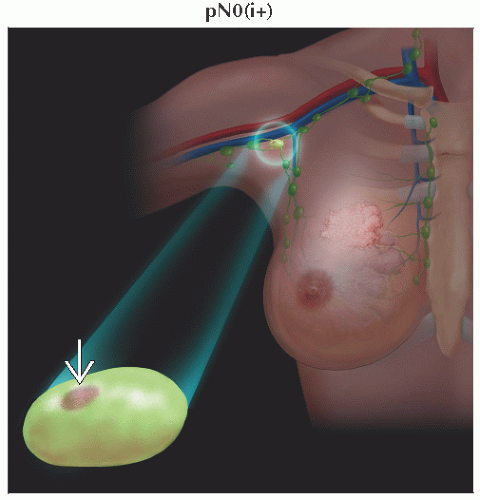
pN0(i-) | No regional lymph node metastases histologically, negative IHC2 | ||
pN0(i+) | Malignant cells in regional lymph node(s) ≤ 0.2 mm (detected by H&E or IHC including ITC3) | ||
pN0(mol-) | No regional lymph node metastases histologically, negative molecular findings (RT-PCR)4 | ||
pN0(mol+) | Positive molecular findings (RT-PCR),4 but no regional lymph node metastases detected by histology or IHC | ||
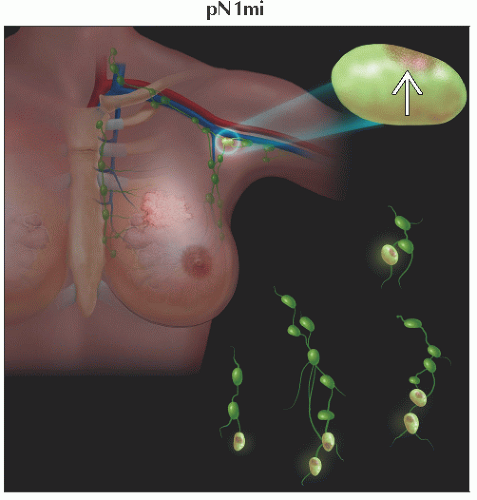
pN1 | Micrometastases; or metastases in 1-3 axillary lymph nodes; &/or in internal mammary nodes with metastases detected by sentinel lymph node biopsy but not clinically detected5 | ||
pN1mi | Micrometastases (> 0.2 mm &/or > 200 cells, but none > 2.0 mm) | ||
pN1a | Metastases in 1-3 axillary lymph nodes, ≥ 1 metastasis > 2.0 mm | ||
pN1b | Metastases in internal mammary nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected5 | ||
pN1c | Metastases in 1-3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected5 | ||
pN2 | Metastases in 4-9 axillary lymph nodes; or in clinically detected6 internal mammary lymph nodes in the absence of axillary lymph node metastases | ||
pN2a | Metastases in 4-9 axillary lymph nodes (≥ 1 tumor deposit > 2.0 mm) | ||
pN2b | Metastases in clinically detected6 internal mammary lymph nodes in the absence of axillary lymph node metastases | ||
pN3 | Metastases in ≥ 10 axillary lymph nodes; or in infraclavicular (level III axillary) lymph nodes; or in clinically detected6 ipsilateral internal mammary lymph nodes in the presence of ≥ 1 positive level I, II axillary lymph nodes; or in > 3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected5; or in ipsilateral supraclavicular lymph nodes | ||
pN3a | Metastases in ≥ 10 axillary lymph nodes (≥ 1 tumor deposit > 2.0 mm); or metastases to infraclavicular (level III axillary lymph) nodes | ||
pN3b | Metastases in clinically detected6 ipsilateral internal mammary lymph nodes in the presence of ≥ 1 positive axillary lymph nodes; or in > 3 axillary lymph nodes and internal mammary lymph nodes with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected5 | ||
pN3c | Metastases in ipsilateral supraclavicular lymph nodes | ||
1 Classification is based on axillary lymph node dissection with or without sentinel lymph node biopsy. Classification based solely on sentinel lymph node biopsy without subsequent axillary lymph node dissection is designated (sn) for “sentinel node.” | |||
(M) Distant Metastases | Adapted from 7th edition AJCC Staging Forms. | ||
TNM | Definitions | ||
M0 | No clinical or radiographic evidence of distant metastases | ||
cM0(i+) | No clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells in circulating blood, bone marrow, or other nonregional nodal tissue that are ≤ 0.2 mm in a patient without symptoms or signs of metastases | ||
M1 | Distant detectable metastases as determined by classic clinical and radiographic means &/or histologically proven > 0.2 mm | ||
AJCC Stages/Prognostic Groups | Adapted from 7th edition AJCC Staging Forms. | ||
Stage | T | N | M |
0 | Tis | N0 | M0 |
IA | T11 | N0 | M0 |
IB | T0 | N1mi | M0 |
T11 | N1mi | M0 | |
IIA | T0 | N12 | M0 |
T11 | N12 | M0 | |
T2 | N0 | M0 | |
IIB | T2 | N1 | M0 |
T3 | N0 | M0 | |
IIIA | T0 | N2 | M0 |
T11 | N2 | M0 | |
T2 | N2 | M0 | |
T3 | N1 | M0 | |
T3 | N2 | M0 | |
IIIB | T4 | N0 | M0 |
T4 | N1 | M0 | |
T4 | N2 | M0 | |
IIIC | Any T | N3 | M0 |
IV | Any T | Any N | M1 |
Notes: M0 includes M0(i+). The designation pM0 is not valid; any M0 should be clinical. If a patient presents with M1 prior to neoadjuvant systemic therapy, the stage is considered IV and remains stage IV regardless of response to neoadjuvant therapy. Stage designation may be changed if postsurgical imaging studies reveal the presence of distant metastases, provided that the studies are carried out within 4 months of diagnosis in the absence of disease progression and provided that the patient has not received neoadjuvant therapy. Post-neoadjuvant therapy is designated with a “yc” or “yp” prefix. Of note, no stage group is assigned if there is a complete pathologic response (CR) to neoadjuvant therapy, for example, ypT0ypN0cM0. 1 T1 includes T1mi. | |||
Cancer of mammary ducts and glands (ductal carcinoma) or of lobules and terminal ducts (lobular carcinoma)
Ductal carcinoma in situ (DCIS)
Entirely confined to duct system of breast
Both smaller ductolobular units and larger extralobular ducts
Noninvasive
Considered direct precursor of invasive ductal carcinoma, with magnitude of risk being variable and dependent on
Histological grade
Lesion size
Age
DCIS increases chance of developing invasive cancer in ipsilateral or contralateral breast
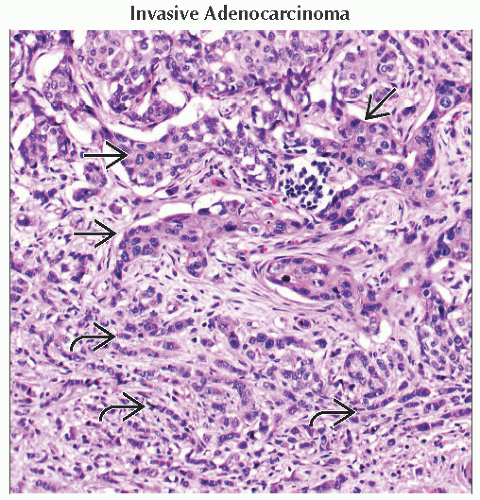
Invasive ductal carcinoma
Most rapidly growing subgroup of breast cancer
˜ 80% of all invasive breast cancers
Lobular carcinoma in situ (LCIS)
Noninvasive lesion that arises from lobules and terminal ducts of breast
Almost always represents incidental finding
Not usually identified clinically, mammographically, or by gross pathologic examination
Not direct precursor lesion for invasive breast cancer, although new studies are challenging this belief
Associated with increased risk of developing invasive ductal or lobular carcinoma in either breast
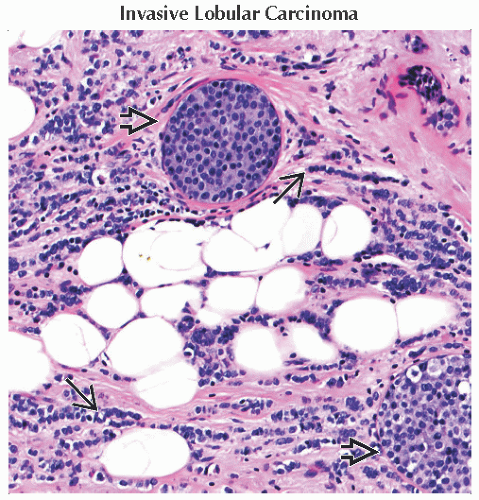
Infiltrating lobular carcinoma
2nd most common type of invasive breast cancer
Accounts for about 5-10% of invasive lesions
Incidence rates of lobular cancer are rising faster than rates of ductal carcinoma in United States
Ductal carcinoma traditionally classified according to architectural pattern
Comedo
Cribriform
Micropapillary
Papillary
Solid
Grade classification
Scarff-Bloom-Richardson scoring system
1-3 points are given for nuclear pleomorphism, mitotic rate, and tubule formation
Low grade: 3-5
Intermediate grade: 6-7
High grade: 8-9
Reflects potential of lesion to recur within breast or to progress to invasive breast cancer
Architectural and cytologic features
Well differentiated (grade 1)
Infiltration of stroma as solid nests of glands
Nuclei are relatively uniform with little or no evidence of mitotic activity
Moderately differentiated (grade 2)
Infiltration as solid nests with some glandular differentiation
Nuclear pleomorphism and moderate mitotic rate
Poorly differentiated (grade 3)
Composed of solid nests of neoplastic cells without evidence of gland formation
Marked nuclear atypia and considerable mitotic activity
Comments
DCIS represents heterogeneous group of proliferative lesions with diverse malignant potential
Proliferation of presumably malignant epithelial cells within mammary ductal system
No evidence of invasion into surrounding stroma on routine light microscopic examination
Risk factors
Gender
Females have 100x higher risk than males
Age
Incidence rates rise sharply with age until about 45-50 years
Benign breast conditions
Multiple nonproliferative lesions with cytologic atypia
Personal history
Invasive or in situ breast cancer
Family history
Dietary factors
Alcohol
Moderate alcohol intake is associated with increased risk of hormone receptor-positive breast cancer
Fat, red meat intake
Smoking
Exposure to endogenous estrogens for prolonged period
Younger age at menarche
For every 2-year delay in onset of menarche, approximately 10% reduction in cancer risk
Later age of menopause
Relative risk increases by 1% for each year older at menopause
Nulliparity
Older age at 1st full-term pregnancy
Women with 1st full-term pregnancy at age 35 have 1.6x higher risk than women who bear 1st child at age 26-27
Long-term use of postmenopausal hormone replacement therapy (HRT)
Ionizing radiation of chest at young age
Number of cases in USA per year
> 229,000 cases expected in USA in 2012
> 39,000 deaths
Widespread adoption of mammographic screening dramatically altered number of cases of DCIS
Increased detection led to increased number of cases
DCIS now accounts for approximately 21% of all new breast cancers diagnosed in USA
Over 90% of new cancers are detected only on imaging studies
Inherited breast-ovarian cancer syndrome defined by mutations in BRCA genes
Mutations in BRCA1 and BRCA2 genes
5-10% of women with family history of breast cancer have germline mutations of BRCA genes
Lifetime risk of developing breast cancer is 40-85%
Common in Jewish ancestry
DCIS occurs at younger age
Other genes related to breast cancer include mutations of
p53
ATM
PTEN
BRCA mutation
Carriers have higher risk of breast and ovarian cancers
BRCA1 carriers have 2-3x increased risk of additional malignancies
Melanoma
Pancreatic
Prostate
Colon
BRCA2 mutation carriers also have increased risk of additional malignancies
Pancreatic
Gastric
Male breast cancer
Melanoma
Lifetime risk of developing breast cancer is 40-85%
Li-Fraumeni syndrome
Autosomal dominant
Multiple tumors associated with syndrome
Soft tissue sarcomas
Osteosarcomas
Leukemias
Brain tumors
Early onset breast cancer
50% of carriers develop some form of cancer by age 30; 90% do so by age 70
CHEK2 mutations
2-3x increased risk of breast cancer
Ataxia-telangiectasia
Autosomal recessive
2x increased risk of breast cancer was identified in many epidemiologic studies of ataxia-telangiectasia families
Cowden syndrome
Rare autosomal dominant
Germline mutations in PTEN
Multiple hamartomas and increased risk of early onset breast and thyroid cancer
Breast cancer develops in 25-50% of female carriers
Peutz-Jeghers syndrome
Autosomal dominant
Relative risk for breast &/or gynecologic cancer in affected women is 20
Hereditary diffuse gastric cancer syndrome
Autosomal dominant
Mutations in E-cadherin gene
Cumulative risk of lobular breast cancer ranges from 20-54%
50% of patients with sporadic lobular breast cancers have E-cadherin mutations
Invasive ductal carcinoma on gross pathologic evaluation
Typically hard, gray-white, gritty masses
Tumor invades surrounding tissue in haphazard fashion to create characteristic irregular, stellate shape
Malignant cells induce fibrous response as they infiltrate breast parenchyma, resulting in
Clinically and grossly palpable mass
Radiologic density
Solid sonographic characteristics
Lobular carcinoma
Firm, irregularly marginated tumors
Often infiltrate to point where tumor margin cannot be identified
H&E
Comedo
Prominent necrosis in center with calcification
Cells are large and show nuclear pleomorphism
Mitotic activity may be prominent
Cribriform
Formation of back-to-back glands without intervening stroma
Small to medium-sized cells with relatively uniform hyperchromatic nuclei
Mitoses are infrequent, and necrosis is limited to single cells or small cell clusters
Micropapillary
Small tufts of cells oriented perpendicular to basement membrane of involved spaces and projecting into lumina
Club-shaped cells
Micropapillae lack fibrovascular cores
Nuclei show diffuse hyperchromasia
Mitoses are infrequent
Papillary
Intraluminal projections of tumor cells demonstrate fibrovascular cores and thereby constitute true papillations
Intracystic papillary carcinoma, a variant of papillary DCIS, is characterized by cells in a single cystically dilated space
Solid
Not as well defined as other subtypes
Tumor cells fill and distend involved spaces and lack significant necrosis, fenestrations, or papillations
Ductal carcinoma
Small epithelial cells in single file growing around ducts and lobules; clusters and sheets also possible
Signet ring cells are frequently seen
Often concentric rings of tumor cells surround normal ducts
Cytology: Small cells containing oval or round nuclei with nonadherent, small nucleoli
Cells of atypical lobular hyperplasia, LCIS, and invasive lobular carcinoma are identical
Primary route of dissemination of breast cancer is via axillary lymphatics
Reported incidence of axillary lymph node involvement in patients with DCIS with microinvasion averages ≤ 5%
Lymph node involvement strongly correlated with tumor size in invasive lesions
Other routes of spread
Supraclavicular node or internal mammary nodes
Direct tumor extension through chest wall
Common sites of metastasis include
Bone
Lung
Liver
Lobular carcinoma
More likely than ductal carcinoma to spread to abdomen
Peritoneum
Retroperitoneum
Gastrointestinal tract
Ovaries
Uterus
Less likely to metastasize to pleura and lungs
Common radiologic presentations
May present with palpable thickening/lump or abnormality seen on mammogram
Classic findings are dense mass with spiculated margins with possible associated calcifications
Mammography and ultrasound establish diagnosis
T1-weighted, post-contrast fat-saturated MR is ideal for mapping extent of disease after diagnosis
Ultrasound
Preferred modality for determining cystic vs. solid nature of mass found on mammography
DCIS
Sensitivity is 50%
Findings include
Dilated ducts
Indistinct walls
Echogenic calcification
On power Doppler, increased vascularity is common
Infiltrating ductal carcinoma
Irregular hypoechoic shadowing mass
Taller-than-wide or perpendicular to skin
Architectural distortion, may have echogenic halo
Mammography
Screening mammography ± clinical breast examination is recommended annually for women age 40 and older
Mass densities and calcification are most sensitive findings
Sensitivity increases during follicular phase of menstrual cycle
Majority of ductal breast carcinoma cases are detected on mammography screening
Microcalcifications have sensitivity of 70-80% for DCIS
Breast imaging-reporting and data system (BI-RADS): Quality assurance reporting system
0: Incomplete
1: Negative
2: Benign finding(s)
3: Probably benign
4: Suspicious abnormality
5: Highly suggestive of malignancy
6: Known biopsy proven malignancy
Mammography
DCIS: Calcifications are more common finding
Fine, linear, or branching calcifications are highly suggestive of DCIS
Most cases of breast cancer detected on screening are stage I
Dense mass with spiculated margins
Focal asymmetric density ± distortion
Associated with calcifications
CT
More useful for assessment of spread than for imaging of primary lesions
CECT
Useful for mediastinal and organ metastases, especially for liver metastases
Malignancy typically measures above water attenuation
When large, appears as discrete mass and may be spiculated
Tumors appear as dense lesions and show early contrast enhancement
NECT
Useful for lung and pleural metastases, detecting lymphatic spread
MR
DCIS: Sensitivity is 88-95%
T1WI C+ FS: Linear or segmental clumped enhancement
Infiltrating ductal carcinoma
T2WI FS: Usually hypointense focal mass
T1WI C+ FS: 90% enhance rapidly and intensely, may have rim enhancement, internal enhancing septations
Useful for evaluation of brain and hepatic metastases
Used in some high-risk patients to look for bilateral breast involvement
PET/CT
Can be used to assess distant metastases, local recurrence, and treatment response
Sensitivity is 80-90% for evaluation of primary tumors
Positive axillary lymph nodes on PET/CT has high positive predictive value for malignancy despite relative insensitivity (80%)
Patients with suspected advanced disease or deemed “high risk” should be considered for PET/CT for overall staging evaluation
Not usually recommended for initial diagnosis but may help in patients with implants or dense breast tissue
80-95% sensitivity for detecting distant metastases at time of initial diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree