The individual has personal or family history features suggestive of genetic cancer susceptibility
The genetic test can be adequately interpreted
The test results will aid in the management of the patient or his/her family members
The American Society of Breast Surgeons position statement on BRCA genetic testing suggests that any of the following features should prompt education and referral to genetic counseling [32]:
Breast cancer diagnosed before the age of 50
Two primary breast cancers in the same person
A family history of breast cancer diagnosed before the age of 50
Breast cancer in a man
Personal or family history of ovarian cancer
Personal or family diagnosis of breast cancer in the setting of Ashkenazi Jewish heritage
A known BRCA1 or BRCA2 mutation within the family
Triple-negative breast cancer diagnosed before the age of 60
Pancreatic cancer associated with family history of breast or ovarian cancer
Statistical models intended to provide a likelihood of having an underlying BRCA1 or BRCA2 mutation have been developed and incorporate the personal and family history variables described above. Available models include BRCAPRO and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm [33, 34].
A detailed and comprehensive family history should be obtained by the clinician considering genetic testing for any patient. In regard to testing for BRCA1 and BRCA2 breast cancer syndromes, it must be recognized that these genes carry not only increased risk for breast cancer, but for other cancers as well. As such, a three-generation family history is recommended, which should elicit details about any cancers diagnosed in relatives, with particular attention to cancers of the breast, ovaries, endometrium, thyroid, adrenal, pancreas, brain, or soft tissues. Furthermore, as BRCA1 and BRCA2 are autosomal dominant and may be inherited from either parent, both maternal and paternal family histories should be thoroughly obtained. When possible, other important details to collect in the family history include age at diagnosis of cancer, risk reduction variables including chemoprevention or prophylactic operations, exposure to carcinogens, hormone and reproductive history, and previous breast biopsies. As these details are sophisticated, verification from medical records is recommended, whenever possible [35].
There are several factors that may render the family history less informative. A small family size, or a low number of the susceptible gender for gender-specific cancers, may decrease sensitivity to detect genetic predisposition syndromes. Furthermore, prophylactic operations that remove the susceptible organs create uncertainty as to whether cancer would have developed in that family member. Deaths at an early age, before the age of onset of inherited cancer syndromes, may limit the utility of the family history. If any of these factors is present, the probability of BRCA1 or BRCA2 mutation may be underestimated [36].
When the decision has been made to pursue genetic testing based on the available personal and family history, the recommended strategy is to test a family member who has already been diagnosed with cancer. This recommendation follows from the fact that a negative test in an unaffected person is rather inconclusive. It would remain unknown whether a gene mutation exists in the family, as the tested unaffected person may simply have not inherited the gene that is indeed present in other family members. Additionally, those who undergo full BRCA1 and BRCA2 gene sequencing may have a result indicating a variant of unknown significance, which indicates a DNA mutation that may or may not confer increased cancer risk.
Furthermore, if an already diagnosed person is tested, and a mutation is revealed, it allows subsequent family members to be tested only for the specific gene mutation already identified as the culprit within the family; therefore, it saves additional family members from full genetic sequencing of BRCA1 and BRCA2. In high-risk ethnic populations, such as Ashkenazi Jewish, Icelandic, and Finnish, who in general harbor increased frequency of specific founder mutations, an accepted strategy is to first test the individual specifically for the founder mutation. If the initial test is negative, proceeding to full sequencing of the BRCA1 and BRCA2 genes is then recommended.
There are several possible outcomes of genetic testing for BRCA1 or BRCA2 mutations. First, a test may indicate the existence of a known mutation causing abnormal function of the BRCA1 or BRCA2 protein. This result indicates an increased risk of breast, ovarian, and other cancers, as discussed previously. Patients with this result should then be managed according to guidelines for those with increased risk, which will include surveillance, chemoprevention, or prophylactic operations. A negative result, indicating no identified mutation of BRCA1 or BRCA2, must be interpreted in the context of personal and family history. In a patient with a known gene mutation in the family, a negative result for that specific mutation will mean that the patient has not inherited the trait; his or her risk for cancer is then considered to be equivalent to the general population and is based on factors recognized to be associated with cancers in established risk estimation models. In a patient already diagnosed with cancer, a negative result may have one of several interpretations. It may indicate that a gene mutation is not present within the family, or that a mutation does exist, but cannot be identified with current sequencing technology. It is also possible that a BRCA mutation may exist within a family, while a tested individual with cancer has a sporadic form of cancer. Finally, genetic testing for BRCA gene mutations may have an indeterminate result. That is, there may be small mutations, located in non-critical gene domains, that have unknown consequences for the functional protein. This result confers an unknown cancer risk.
In those already diagnosed with cancer and suspected of having a genetic predisposition syndrome, testing should be considered before definitive treatment plans have been decided, according to the patient’s preferences. The result of the genetic test may inform the decisions regarding treatment options. For example, a woman may use the information to decide whether to pursue breast conservation or mastectomy on the affected side and may additionally consider contralateral prophylactic mastectomy based on the results of the genetic testing. However, some patients may elect to proceed with treatment for the primary cancer without genetic testing results.
The implication of genetic testing for hereditary cancer on health insurance coverage has been a concern of patients and may contribute to anxiety surrounding the test. In general, genetic test results are protected from disclosure to health insurance providers. It is illegal in the United States to deny insurance coverage based on genetic testing results and similarly illegal to consider genetic information as a preexisting condition, owing to protections set forth in the Health Insurance Portability and Accountability Act (HIPAA) of 1996. Furthermore, the federal Genetic Information Nondiscrimination Act (GINA) of 2008 prohibits discrimination based on genetic test results. Patients can generally be reassured that there is little risk to future health insurance coverage based on genetic testing results alone; however, insurance providers may inquire about cancer diagnoses within the family and the individual.
For many years, a company known as Myriad Genetics held patents making it the exclusive provider of BRCA1 and BRCA2 genetic testing in the United States. The tests were costly, at up to $4,000, which may have restricted access for some patients. In 2013, the Supreme Court issued a ruling in Association for Molecular Pathology v. Myriad Genetics, Inc., which changed the landscape of genetic testing, not only for BRCA1 and BRCA2 but with broad implications for cancer diagnostics in general. In essence, the court justices determined that human genes are not eligible for patents. Their decision was based upon the premise that DNA sequences are naturally occurring, and merely sequencing a naturally occurring phenomenon is not adequately inventive to deserve a patent. Consequently, the patent held by Myriad, which provides a result by comparing an individual’s isolated BRCA1 and BRCA2 sequences to reference sequences, was invalidated. Since that decision, a number of competitors have entered the market and will offer BRCA1 and BRCA2 diagnostic genetic testing. This competition in the market will lead to lower prices for these tests and may improve access for certain patients [37, 38].
Genetic Counseling
Referral for genetic testing must be preceded by detailed counseling and informed consent. In particular, the American Society of Breast Surgeons recommends a discussion of several relevant issues. Patients must understand the possible outcomes, which may include positive, negative, and inconclusive results. It is important to understand that these results will have different implications for different patients. For example, those with a family history of a known BRCA gene mutation can interpret negative results as essentially eliminating the possibility of a genetic predisposition syndrome, while those without previously identified mutation within the family should understand that a negative result will not definitively exclude the possibility of a mutation that is not detectable but may be clinically significant. The possibility of an indeterminate result must also be discussed. Patients should be educated as to the cancer risks associated with mutations in BRCA1 and BRCA2, along with medical and surgical management options. These options will include increased surveillance, chemoprevention, and prophylactic operations. There must also be an understanding that the result of genetic testing will have implications for the patient and for his or her family. A discussion of disclosure of genetic information to family, and possible testing of family members if the result is positive, should occur before testing is pursued.
In general, any consideration of genetic testing for cancer susceptibility genes should be done with the guidance of certified genetic counselors. The critical elements of genetic counseling, as defined by the National Society of Genetic Counselors, are (1) to discuss the risks, benefits, and limitations of genetic counseling (including details about sensitivity, specificity, inconclusive results, and results of unknown clinical significance), (2) to discuss cancer prevention and management, (3) to discuss psychosocial support, and (4) to provide information tailored to each patient’s level of understanding [39, 40].
In order to provide the most beneficial genetic counseling, providers must have a detailed understanding of the patient’s demographic and educational background. An assessment of the psychological and emotional state of the patient is also critical. In particular, it is valuable to understand the emotional well-being of the patient by assessing for symptoms of anxiety and depression and inquiring about mental health history. The emotional response to cancer diagnoses within the family can help identify patients who may experience more distress with results of genetic testing. Understanding the coping skills and strategies of the individual may similarly provide important data. Any concerns about the psychological health of the patient may warrant involvement of a mental health professional as part of the multidisciplinary team caring for the unique set of individuals considering testing for genetic predisposition syndromes [39].
In younger patients with BRCA1 or BRCA2 gene mutations, issues of reproductive options may become a concern. Genetic counselors can provide advice and support related to reproductive options, should potential parents choose to limit their risk of passing a deleterious mutation to their children. Reproductive technologies, such as preimplantation diagnosis, are available as an option to concerned individuals [41].
Management Options for Patients with Brca1/2 Mutations
Management of women with BRAC1 or BRCA2 mutations is based upon vigilant screening and preventive measures such as chemoprevention or prophylactic operations.
Vigilant Screening Recommendations
Screening guidelines are published by the National Comprehensive Cancer Network [31]. These guidelines apply to individuals with a known personal or family mutation of BRCA1 or BRCA2. Notably, individuals who have not undergone genetic testing but who have a family member with a known BRCA1 or BRCA2 mutation should be managed according to the guidelines for patients with a confirmed genetic mutation. That is, even the suspicion that the patient may harbor a deleterious mutation of BRCA1 or BRCA2 is enough to pursue management as if the patient has the confirmed syndrome.
Screening for Breast Cancer
In patients with known or suspected BRCA1 or BRCA2 mutations, screening should begin at age 25 or earlier based upon the earliest age at diagnosis within the family. Breast awareness and self-exam should be taught and patients instructed to perform breast self-exam every month. Clinical breast examination is recommended every 6–12 months starting at age 25 or individualized based on the earliest age of onset in the family. Patients should also be counseled regarding the risk and benefits of prophylactic mastectomy, prophylactic bilateral salpingo-oophorectomy, and chemoprevention options for breast and ovarian cancers (Table 6.2) [31]. Mammography and breast magnetic resonance imaging (MRI) are indicated yearly [30].
Table 6.2
Vigilant screening for breast cancer in BRCA1/2 mutation carriers
Breast awareness starting at age 18 years |
Starting at age 25 years, clinical breast exam every 6–12 months |
Annual mammography and breast MRI starting at age 25 years or individualized based on the earliest age of onset in the family |
Discuss risk-reducing mastectomy |
Recommend prophylactic bilateral salpingo-oophorectomy |
Consider chemoprevention options for breast and ovarian cancers |
The use of MRI as a screening test remains somewhat controversial. MRI is useful among younger women with dense breasts, who are particularly at risk in the setting of BRCA1 and BRCA2 mutations, which cause cancers at a younger age; in this population, the test is more sensitive to detect breast cancer than mammography, ultrasound, or clinical breast exam [42, 43]. The American Cancer Society does endorse breast MRI as a screening test in those with a genetic predisposition syndrome, since the sensitivity of mammography may be as low as 33 % in these younger women with dense breasts [44, 45]. A study of alternating mammography and breast MRI every 6 months among 73 patients with BRCA1 or BRCA2 mutations showed that 12 of 13 cancers identified on MRI were not seen on the preceding mammogram 6 months earlier [46]. Additionally, the prospectively studied efficacy of a screening protocol that includes yearly breast MRI showed the strategy to have high sensitivity to detect cancers at an early stage, with excellent long-term survival outcomes [47]. However, it must be acknowledged that MRI may detect benign lesions that cannot be easily distinguished from invasive cancer by this modality. Therefore, MRI may lead to additional biopsies if used as a screening tool.
Guidelines for men with BRCA1 or BRCA2 mutations are similar to those for women; they include monthly breast self-exam, semiannual clinical breast exam, and mammography if gynecomastia is present. Screening for prostate cancer should follow population guidelines, which recommend yearly digital rectal examination and prostate-specific antigen (PSA) serology.
Screening for Ovarian Cancer
Regarding the increased risk of ovarian cancers in women carrying mutations of BRCA1 or BRCA2, twice yearly pelvic examinations should be performed starting at age 30 or 5–10 years before the earliest age of first diagnosis of ovarian cancer. Along with biannual pelvic examination, CA-125 serology and transvaginal ultrasound should be performed (Table 6.3) [31]. However, because early detection is often difficult despite screening, and because of the poor prognosis associated with advanced ovarian cancer, prophylactic bilateral salpingo-oophorectomy is the recommended management strategy for these patients, as addressed later in this chapter.
Table 6.3
Vigilant screening for ovarian cancer in BRAC1/2 mutation carriers in those who have not elected prophylactic bilateral salpingo-oophorectomy
Every 6 months, starting at age 30 years or 5–10 years before the earliest age of first diagnosis of ovarian cancer in the family |
Pelvic examination |
Transvaginal ultrasound |
Serum CA-125 |
Chemoprophylaxis
Chemoprophylaxis has been studied in the context of BRCA1 and BRCA2 mutations. In women with a personal history of breast cancer, tamoxifen has been shown to reduce contralateral breast cancer risk by 50 % in BRCA1 and BRCA2 mutation carriers. Interestingly, such risk reduction occurs regardless of the tumor’s receptor status, especially given that BRCA1-associated breast cancers are more often estrogen receptor negative [48–50]. Of note, although chemoprophylaxis for BRCA1 and BRCA2 mutation carriers has been shown to potentially reduce the risk of developing contralateral breast cancer, it has not been shown to significantly reduce overall mortality.
Oral contraceptives may have some protective effect for ovarian cancer in BRCA1- and BRCA2-positive patients; some studies have suggested a risk reduction as high as 60 % with oral contraceptive use of 6 years duration [51].
Prophylactic Surgery
Contemplation of prophylactic operations for breast and ovarian cancers is of particular importance among individuals carrying mutations of BRCA1 and BRCA2. Prophylactic operations are highly effective in preventing cancer; however, surgical prevention may be considered aggressive and is associated with medical and psychosocial considerations.
The available options are prophylactic bilateral, or risk-reducing, mastectomy in patients with no cancer diagnosis [52–55], therapeutic mastectomy with contralateral prophylactic mastectomy in those with a cancer diagnosis [5, 56], and finally, prophylactic bilateral salpingo-oophorectomy (PBSO) performed alone or in addition to mastectomy in patients with or without cancer [53, 57–60]. Regardless, there is a lack of data to suggest an overall survival advantage with prophylactic bilateral mastectomy for BRCA1 and BRCA2 mutation carriers, whereas there are data that demonstrate a survival advantage with PBSO [53].
Impact of Prophylactic Bilateral Mastectomy for BRCA1 and BRCA2 Carriers
Prophylactic bilateral mastectomy significantly reduces the risk of developing breast cancer in BRCA1 and BRCA2 mutation carriers [52–55] (Table 6.4). The Prevention and Observation of Surgical Endpoints (PROSE) study is an often-quoted examination of the risk reduction afforded by prophylactic bilateral mastectomy. The study prospectively followed 105 BRCA1 or BRCA2 carriers who elected to have prophylactic bilateral mastectomy and compared these to 378 matched BRCA1 or BRCA2 carriers who did not choose prophylactic bilateral mastectomy. Mean follow-up was 6.4 years. Breast cancer was ultimately diagnosed in only two women who had prophylactic bilateral mastectomy (1.9 %), while 184 women in the conservatively managed group developed breast cancer (48.7 %). The experimental design of this study accounted for prophylactic salpingo-oophorectomy as well. The authors conclude that prophylactic bilateral mastectomy provides a relative risk reduction of 90 % in women who have ovaries and of 95 % in women who have also undergone bilateral salpingo-oophorectomy. The absolute risk reduction was 46.8 % [55].
Table 6.4
Selected series of impact of prophylactic bilateral mastectomy for BRCA1/2 mutation carriers
Authors, year, ref. | N | Results | Conclusions |
|---|---|---|---|
Meijers-Heijboer, 2001 [52] | 139 | Incidence of breast cancer | Prophylactic mastectomy reduces incidence of breast cancer |
0 %: prophylactic group | |||
2.5 %: surveillance group | |||
Hartmann, 2001[54] | 26 | Incidence of breast cancer | Breast cancer risk reduction in prophylactic group: 90–100 % |
0 %: prophylactic group | |||
Rebbeck, 2004 (PROSE) [55] | 483 | Incidence of breast cancer 1.9 %: prophylactic group 49 %: surveillance group | Breast cancer risk reduction in prophylactic group: 90 % |
No impact on OS | |||
Domchek, 2010 [53] | 2,482 | Incidence of breast cancer | Prophylactic mastectomy reduces incidence of breast cancer |
0 %: prophylactic groupa | |||
5.8–8.1 %: surveillance groupb |
Another long-term study of 26 women with BRCA1 or BRCA2 mutation who underwent prophylactic bilateral mastectomy showed no incidence of breast cancer after an average of 13.4 years of follow-up [54]. While the magnitude of risk reduction afforded by prophylactic bilateral mastectomy is dramatic, there have been no randomized clinical trials to further define the efficacy, utility, and risk when compared with more conservative management of high-risk patients. Therefore, consideration of prophylactic bilateral mastectomy remains a largely personal and often difficult decision for the patient.
Domchek et al. estimated the risk and mortality reduction following prophylactic surgery by analyzing data from a prospective, multicenter cohort study of 2,482 women with BRCA1 or BRCA2 mutation [53]. For women who had prophylactic mastectomy, either with or without prophylactic salpingo-oophorectomy, the number of breast cancer events observed during the 3 years of prospective follow-up was zero. In contrast, for women who did not undergo a prophylactic mastectomy, the incidence of developing breast cancer was 8.1 % in those who had prophylactic salpingo-oophorectomy and 5.1 % in those who did not [53]. Thus, this confirms that prophylactic bilateral mastectomy was associated with a decreased risk of breast cancer in BRCA1 and BRCA2 mutation carriers.
Despite the risk reduction afforded by prophylactic bilateral mastectomy, there are no prospectively collected data to determine whether the decreased incidence of breast cancer is associated with improved overall survival. Several studies have used theoretical modeling to study long-term survival associated with prophylactic bilateral mastectomy and with prophylactic bilateral salpingo-oophorectomy. These models suggest increases in survival of 2.9–5.3 additional life years with prophylactic bilateral mastectomy, and 0.3–1.7 years with prophylactic bilateral salpingo-oophorectomy, when the prophylactic procedures are performed at the age of 30 years [61, 62]. Notably, the survival benefit of the prophylactic procedures decreases when performed later in life; in these models, there were negligible years gained from prophylactic operations after the age of 60 years.
Psychological and quality of life concerns are essential considerations when discussing prophylactic bilateral mastectomy. Studies have shown that most women are content with the decision to undergo prophylactic surgery, and satisfaction rates as high as 95 % have been reported [63]. Dissatisfaction, although rare, was more common when a physician’s advice was the motivating factor to pursue prophylactic surgery, rather than the initiative of the patient [64]. Sources of anxiety in dissatisfied women were related to cosmetic outcome, reconstruction outcome, body image and sexuality, and fear of developing cancer despite the prophylactic operation. Quality of life seems to be similar when comparing women who have undergone prophylactic mastectomy and those at high risk of breast cancer who have not elected prophylactic surgery. Poorer quality of life in the study subjects was self-reported and appeared unrelated to issues surrounding prophylactic mastectomy, with issues of depression and poor general health being the most important contributors [65].
Impact of Contralateral Prophylactic Mastectomy in Women with History of Invasive Breast Cancer
Women with a diagnosis of unilateral breast cancer in the setting of BRCA1 or BRCA2 gene mutations may elect to have contralateral prophylactic mastectomy. Studies have shown risk reduction for breast cancer to be similar to that afforded by bilateral prophylactic mastectomy [5, 56] (Table 6.5). One study that compared BRCA1 and BRCA2 mutation carriers with stage I–IIIa breast cancer who underwent contralateral mastectomy to those who opted for surveillance alone reported a 91 % risk reduction in the contralateral prophylactic mastectomy group [56]. However, no long-term survival benefit was evident in the contralateral prophylactic mastectomy group; rather, a survival benefit was demonstrated from bilateral salpingo-oophorectomy. The mean follow-up time was 3.5 years and the authors believed that longer follow-up is needed to have a better understanding of the effect of contralateral prophylactic mastectomy on contralateral breast cancer-specific survival.
Table 6.5
Selected series of impact of contralateral mastectomy for BRCA1/2 mutation carriers with personal history of breast cancer
Authors, year, ref. | N | Results | Conclusions |
|---|---|---|---|
Metcalfe, 2004 [5] | 491 | Incidence of developing contralateral breast cancer | CPM significantly reduces risk of developing contralateral breast cancer |
0.7 %: CPM group | |||
28.8 : surveillance group | |||
van Sprundel, 2005 [56] | 148 | Overall survival | CPM significantly reduces risk of developing contralateral breast cancer by 91 %, irrespective of the effect of prophylactic oophorectomy |
94 %: CPM group | |||
77 %: surveillance group | |||
No differences in the overall survival between CPM and surveillance groups |
Another study of BRCA1 and BRCA2 mutation carriers with stage I–II breast cancer who were followed for an average of 9.2 years showed that only one cancer developed among 146 women who underwent contralateral prophylactic mastectomy; this translates to a rate of 0.7 % for developing contralateral breast cancer. This was in contrast to a rate of 28.8 % for developing contralateral breast cancer in women who did not undergo contralateral prophylactic mastectomy, with 97 cancer recurrences among this group of 336 women [5].
Long-term satisfaction with contralateral prophylactic mastectomy is reported to be high [66]. Contralateral prophylactic mastectomy is considered to be cost-effective in women younger than 70 years of age when compared with screening, based upon evidence from a study that employed a Markov model analysis [67].
Options for prophylactic mastectomy, whether bilateral or contralateral, include traditional total mastectomy, skin-sparing mastectomy, or nipple-sparing or subcutaneous mastectomy. Total or simple mastectomy generally entails the removal of the breast, along with the nipple-areolar complex and a significant amount of skin overlying the breast tissue (Fig. 6.1). In a skin-sparing mastectomy, a small portion of the skin including the nipple-areolar complex is removed along with the underlying breast tissue while leaving behind a significant amount of skin for reconstruction (Figs. 6.2, 6.3, and 6.4). In the nipple-sparing mastectomy, the entire skin overlying the breast including the nipple-areolar complex is preserved while the breast tissue underneath the skin is removed (Fig. 6.2).
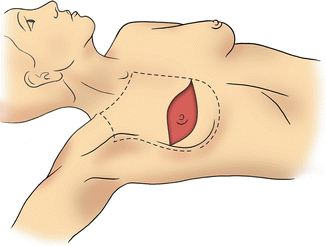
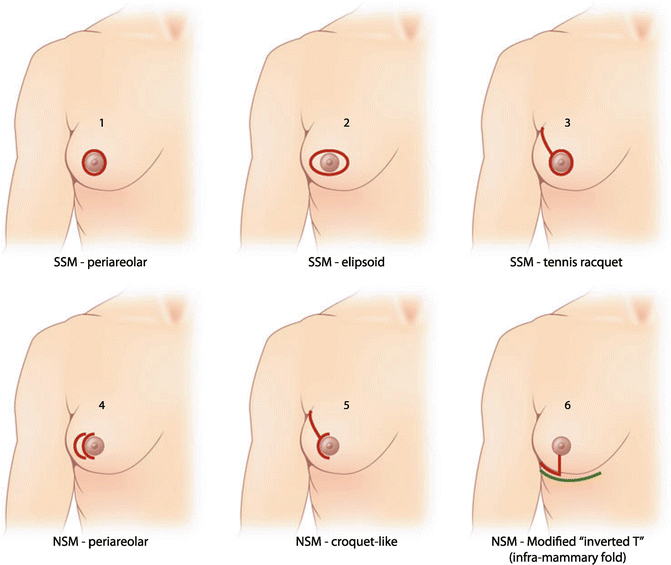
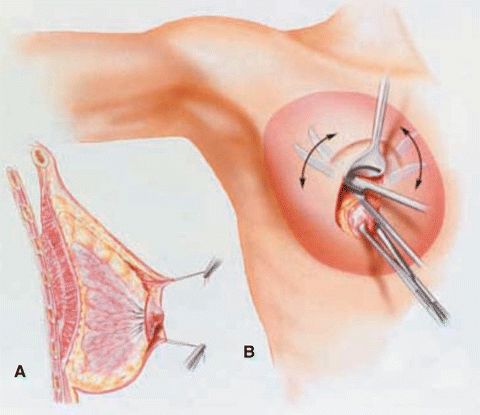
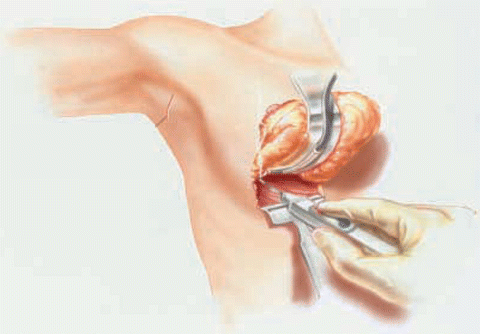

Fig. 6.1
Total or simple mastectomy: Dotted lines represent the extent of breast tissue to be removed. Solid red area represents the classic mastectomy incision, which incorporates the nipple-areolar complex and a portion of skin over the breast (Courtesy of Quyen D. Chu, MD, MBA, FACS)

Fig. 6.2
Possible incisions for skin-sparing mastectomy (SSM) and nipple-sparing mastectomy (NSM) (Reprinted from Max Dieterich, Bernd Gerber. Patient selection and technical considerations in nipple-sparing and areola-sparing mastectomy. Current Breast Cancer Reports 2011;3(2): 79–87. from Springer Verlag)

Fig. 6.3
Techniques of a skin-sparing mastectomy. The nipple-areola complex and the underlying breast tissue are included in the specimen. Skin flaps are elevated circumferentially above the breast tissue (Modified from Toth B, Daane S, Tenna S. Skin-sparing mastectomy with immediate breast reconstruction: a 10-year, single surgeon review of 105 consecutive patients. Eur J Plast Surg 2002;25:156–9. With permission from Springer Verlag)

Fig. 6.4
Breast tissue is dissected away from the chest wall (Modified from Toth B, Daane S, Tenna S. Skin-sparing mastectomy with immediate breast reconstruction: a 10-year, single surgeon review of 105 consecutive patients. Eur J Plast Surg 2002;25:156–9. With permission from Springer Verlag)
Both total mastectomy and skin-sparing mastectomy are acceptable options since they have similar oncologic outcome, although the latter tend to result in a better cosmetic outcome [68]. The question of whether or not nipple-sparing mastectomy is safe and appropriate as prophylaxis in those with genetic predisposition syndromes is currently debated. A study of mastectomy specimens from BRCA1 and BRCA2 mutation carriers showed that premalignant or malignant lesions of the nipple-areolar complex occurred in 10 % of therapeutic mastectomy specimens, but in none of the prophylactic mastectomy specimens. These findings suggest that nipple-sparing mastectomy may be safe to perform as a prophylactic procedure; however, long-term outcomes are unknown and further research is needed [69].
The question of whether or not to perform sentinel lymph node biopsy at the time of prophylactic mastectomy is controversial. 3.5–5 % of all prophylactic mastectomy specimens contain occult invasive cancers [70, 71]. Once mastectomy is complete, and the opportunity to perform sentinel lymph node biopsy is lost. If sentinel lymph node biopsy is not performed during the prophylactic mastectomy and occult cancer is discovered, the only option remaining for axillary lymph node sampling is complete axillary lymph node dissection, with its attendant morbidities. Some authors have argued in favor of routine axillary lymph node biopsy at the time of prophylactic mastectomy [70], while others have suggested the procedure to be of little value in this setting [72]. Preoperative breast MRI may be a useful adjunct to identify occult cancers before proceeding with prophylactic mastectomy and may help select patients in whom sentinel lymph node biopsy should be performed [73].
Certainly, prophylactic mastectomy does afford a dramatic benefit in breast cancer prevention; it must be emphasized that even this aggressive treatment does not entirely eliminate the possibility of future breast cancer. Mastectomy cannot eliminate every breast tissue cell within the body, and any remaining breast tissue will contain the germline mutation in BRCA1 and BRCA2 carriers. There are many published accounts of breast cancer following prophylactic mastectomy [74–77]. This fact underlies the importance of continued vigilance even after risk-reducing surgery in BRCA1 and BRCA2 mutation carriers. At this time there are no generally accepted best practice guidelines for routine screening in these patients after prophylactic mastectomy; however, routine follow-up with clinical examination at least yearly is a prudent strategy [78].
Impact of Prophylactic Bilateral Salpingo-Oophorectomy (PBSO)
Prophylactic bilateral salpingo-oophorectomy (PBSO) is recommended for women with BRCA1 or BRCA2 mutations since there is a lack of efficacy in screening for ovarian cancer. The operation should be performed between the ages of 35 and 40 or at the conclusion of childbearing. Notably, PBSO reduces the risk of ovarian cancer by 96 %, irrespective of whether or not the patient has a personal history of breast cancer [53, 57–60] (Table 6.6). PBSO alone can provide up to 50 % breast cancer risk reduction in the setting of BRCA1 or BRCA2 mutations; such reduction is generally observed in younger, premenopausal women [10, 60]. However, PBSO does not appear to reduce the risk of second diagnosis of primary breast cancer in patients with a personal history of breast cancer [53] (Table 6.6).
Table 6.6
Impact of prophylactic bilateral salpingo-oophorectomy (PBSO) for BRCA1/2 mutation carriers
Authors, year, ref. | N | Results | Conclusions |
|---|---|---|---|
Kauff, 2002 [60] | 170 | Proportion who are disease-free from breast or gynecologic cancers | PBSO decreases risk of breast and gynecologic cancers |
94 %: PBSO group | |||
69 %: surveillance group | |||
Eisen, 2005 [57] | 3,305 | Breast cancer risk reduction following oophorectomy | Oophorectomy significantly reduces risk of developing breast cancer for BRCA1/2 carriers |
BRCA1: 56 % | Risk reduction is higher if oophorectomy was performed before age 40 | ||
BRCA2: 46 % | |||
Finch, 2006 [58]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|