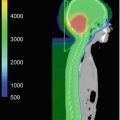
Fig. 13.1
(a) A midbrain JPA in a 6-year-old boy who presented with a right VI nerve deficit and a right hemiparesis. (b) Macroscopic residual disease following initial surgery was resected at a second surgery 2 months after the first. (c) MRI 2 months after the second surgery shows residual signal abnormality in the tumor bed interpreted as possible residual tumor vs. early gliosis. He will continue to be followed with MRI Q3–4 months. If and when there is evidence of progressive tumor, further surgery or systemic chemotherapy would be considered as the next step. Radiotherapy would not normally be considered the best option at this age
13.2.3 Chemotherapy
As for LGG at other locations, chemotherapy has become the treatment of choice in North America for patients less than 10 years of age with a focal brainstem tumor with symptomatic inoperable disease or disease progressive after surgery (Ater et al. 2012; Bouffet et al. 2012; Gururangan et al. 2002, 2007, 2014; Laithier et al. 2003; Packer et al. 1988, 1997, 2009; Prados et al. 1997; Reddy and Packer 1999). Three regimens—vincristine/carboplatin, thioguanine/procarbazine/lomustine/vincristine (TPCV), and weekly vinblastine—have been prospectively assessed in chemotherapy-naïve LGG. Response rates range from 13–70% but event-free survival (PFS), defined as the need to consider another intervention to control tumor progression, does not exceed 50% at 5 years. For children who fail first-line therapies, other drugs including temozolomide, oral etoposide, and VP-16 have been used in small numbers of patients with variable benefit. A regimen combining a chemotherapeutic agent irinotecan and an anti-angiogenic agent bevacuzimab that targets the vascular proliferation often present in these tumors has also been used with some success: a recent phase II study showed an 85% response rate at 6 months, albeit with a similar PFS of 47.8% at 2 years. Many children will have several lines of therapy over the course of their disease. The excellent survival rate of over 95% at 10 years calls into question the benefit of such interventions and underscores the need for a long-term strategy for this patient population, the goal of which would be to minimize risk associated with treatment (not only chemotherapy but also surgery and radiotherapy) and maximize neurological and functional outcomes and quality of life.
13.2.4 Radiotherapy
Radiotherapy may be considered at the time of progression following surgery or chemotherapy or for a tumor inoperable because of location. Guidelines for radiotherapy (target volumes, technique, dose, and fractionation) are as for low grade gliomas in other locations. The probability of tumor control is at least 50–70% (Combs et al. 2009; Merchant et al. 2009) and the risk of long-term morbidity, including neurocognitive sequelae, should be lower now than in the past using modern radiotherapy treatment techniques with image guidance and smaller safety margins.
13.2.5 Evolution of Practice and Future Directions
Surgical resection is more commonly used now than in the past, and chemotherapy has become accepted as a useful and often now the favored approach for patients with progressive inoperable disease. However, radiotherapy should always be considered an option for patients with focal brainstem tumors progressive after surgery or chemotherapy. Like surgery and chemotherapy, radiotherapy practice is evolving. Safety margins are smaller than those used in the past and this, as well as improved techniques and new modalities such as protons that allow better sparing of surrounding uninvolved tissues, reduces the risk of long-term sequelae associated with radiotherapy. Recent molecular breakthroughs using next generation sequencing technologies have shown that JPA have a single oncogenic hit affecting the MAPK pathway and implicating the BRAF oncogene, a key regulator of the MAPK pathway, and the AKT/mTOR pathway. This has opened up promising new avenues for targeted therapy and many agents are now under investigation.
13.3 Diffuse Intrinsic Pontine Gliomas
Diffuse intrinsic pontine gliomas (DIPG) account for approximately half of all tumors arising in the brainstem. The median age at presentation is approximately 7 years. Boys and girls are approximately equally affected. There are no known genetic associations or predisposing factors.
Patients usually present with symptoms of short duration—classically defined as less than 6 months but more usually much shorter—consisting of multiple, often bilateral, cranial nerve deficits (especially cranial nerves VI and VII), long tract signs, and ataxia. MRI is the imaging modality of choice and findings are very characteristic. With the epicenter in the pons with axial and/or exophytic growth in about two-thirds of patients, the classic description is of a tumor that causes diffuse enlargement of the brainstem, often encases the basilar artery, and appears hypointense on T1-weighted sequences and hyperintense on T2, FLAIR without enhancement with gadolinium injection (Fig. 13.2). However, a Pediatric Brain Tumor Consortium study found enhancement in 62% of cases, albeit small volume with a median enhancing volume of 1.28 cc (Poussaint et al. 2011), and a European multinational study that included 316 patients reported ring enhancement in 36% of patients at diagnosis (Jansen et al. 2015). There may be cystic necrosis within the tumor and/or intratumoral hemorrhage. Atypical clinical and/or imaging findings should prompt consideration of other tumor types that are occasionally seen in the brainstem such as atypical teratoid/rhabdoid tumor or PNET and of biopsy for confirmation of diagnosis.
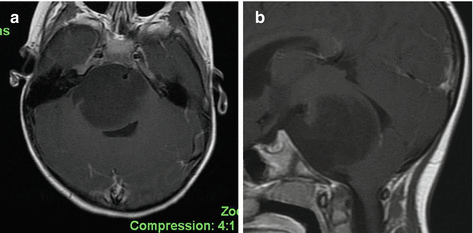

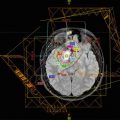
Fig. 13.2
(a Axial, b sagittal) A typical DIPG in a 4½-year-old girl with a 1-week history of facial asymmetry. Note diffuse enlargement of the pons and exophytic growth surrounding the basilar artery. The tumor is hypointense on T1-weighted sequences, hyperintense on T2, FLAIR, and shows no enhancement with gadolinium
13.4 Workup
In addition to MRI of the brain, patients with any symptoms concerning for spinal metastases should have an MRI of the spinal axis to rule out leptomeningeal spread that is seen at diagnosis in about 5% of cases. It is not necessary, and in fact potentially risky, to perform a lumbar puncture for CSF cytology, and, outside the context of a clinical trial with particular requirements, it is not necessary to perform any other studies except routine blood work.
13.5 Acute Management
Steroids will usually be started at presentation and typically result in some improvement in the symptoms and clinical findings. Hydrocephalus is present at diagnosis in less than 10% of patients but if present CSF diversion may be necessary.
13.6 Treatment
In the context of a classical presentation and typical imaging findings, biopsy is not considered necessary for diagnosis—even though now associated with only a low risk of complications—and there is no role for more aggressive surgery. Most commonly, patients are referred on a semi-urgent basis for radiotherapy which is the mainstay of treatment for DIPG.
13.6.1 Radiotherapy
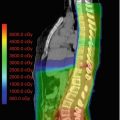
Patients are usually treated supine in a custom-made immobilization device. All patients should undergo CT simulation and for all patients the diagnostic MRI should be co-registered with the CT images. The target volume for radiotherapy is then contoured on MRI. The gross target volume is almost always best defined on T2-weighted or FLAIR MRI (maximum extent of disease). The margin for the clinical target volume is 1–1.5 cm, anatomically constrained by bone and sometimes too by the tentorium. The planning target volume will be technique- and institution specific. Although these days more sophisticated techniques such as IMRT are commonly used, 2D or 3D techniques may also be very acceptable, especially if to do so reduces the preparation time and so the delay to the start of treatment. The radiotherapy dose is typically 54–55.8 Gy given in 30–31 daily fractions of 1.8 Gy over 6 weeks. Alternative fractionation schedules have been extensively investigated in patients with DIPG. Hyperfractionated radiotherapy using fraction sizes of 1–1.26 Gy given twice a day to higher total doses of up to 78 Gy in an attempt to improve tumor control was tested in series of studies in North America in the 1980s and 1990s. There was no evidence of benefit and at the highest doses levels of 76–78 Gy considerable morbidity such as protracted use of steroids and vascular events was observed (Freeman 1996). Accelerated radiotherapy using conventional fraction size of 1.8 Gy given twice a day to total doses of 48.6–50.4 Gy over a shorter overall treatment time of approximately 3 weeks theoretically reduces the opportunity for tumor repopulation. While outcome in a British study was not improved, treatment was well tolerated and considered advantageous in that the patient and family spends less time in/near to the hospital (Lewis et al. 1997). Hypofractionated radiotherapy has been given with the goal of achieving equivalent tumor control with fewer hospital visits. When compared with conventional radiotherapy in previously treated patients and in one prospective randomized study, hypofractionated radiotherapy using doses of 39–45 Gy given once daily with fraction sizes of 3 Gy appeared safe and equally effective and thus could be considered an acceptable option given the shorter overall treatment time of less than 3 weeks and lesser burden for patients and families (Janssens et al. 2013; Negretti et al. 2011; Zaghloul et al. 2014).
13.6.2 Chemotherapy
Chemotherapy has no established role in the treatment of newly diagnosed DIPG. Two randomized phase III trials as well as many phase I/II studies using chemotherapy and/or biological agents concurrent with standard radiotherapy, e.g., cisplatinum, VP-16, temozolomide, topotecan, gadolinium texaphrin, have all failed to show any improvement in outcome (Hargrave et al. 2006). Nimotuzumab, an anti-EGFR monoclonal antibody, appears somewhat more promising with a very high response rate of 96% and a median time to progression of 8.5 months when given in combination with Vinorelbine and radiotherapy (Massimino et al. 2014).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree