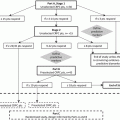
Fig. 10.1
The vicious cycle of bone metastases
Transforming growth factor beta (TGFβ) is released from resorbed bone matrix and has been shown to augment signaling through parathyroid hormone-related protein (PTHrP) [26]. Prostate cancer cells metastatic to bone express PTHrP [27]. Parathyroid hormone receptor is expressed by osteoblasts and stromal cells and signaling through this receptor leads to upregulation in RANKL [28, 29]. NF-κB upregulates expression of PTHrP and RANKL by prostate cancer cells [30]. Calcium is released from resorbed bone. Prostate cancer cells express the calcium-sensing receptor (CaSR) and calcium has been shown to induce proliferation of prostate cancer cells [31]. Thus, TGFβ and calcium are among the growth factors released from bone via osteolysis which fuel cancer cell release of PTHrP, which mediates generation of osteoclastic factors thereby driving the “vicious cycle.”
The bone matrix contains many additional growth factors including fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and Insulin-like growth factor 1 (IGF1) [39]. These and other growth factors likely contribute to prostate cancer growth in the bone microenvironment [40]. Many other molecules have been implicated in the “vicious cycle.” These include endothelin 1 [32], the bone morphogenic proteins (BMPs) [33], Wnt signaling and an inhibitor of Wnt signaling, DKK-1 [34, 35], hypoxia-inducible factor 1 (HIF1) [36], tumor necrosis factor alpha (TNFα) [37], and urokinase-type plasminogen activator (uPA) [38].
SREs in Advanced Disease
As previously stated, bone metastases in advanced prostate cancer are very common. As one might expect, disease metastatic to bone is frequently associated with SREs. Approximately one-half of patients with mCRPC to bone will experience an SRE in a 2-year period in the absence of bone-targeted therapy and 33 % will require radiation to bone, 25 % will experience a pathologic fracture and 8 % will have spinal cord compression [39]. The consequences of SREs to patients are significant. The health-related quality of life (HRQOL) as determined by the Functional Assessment of Cancer Therapy-General (FACT-G) scale shows a decrease in total score and a decrease in subscale scores measuring physical, emotional, and functional well-being in patients with prostate cancer having experienced an SRE compared to patients not having experienced an SRE [40]. Patients having experienced an SRE also report worse pain as determined by scores of the Brief Pain Inventory (BPI) [40]. Perhaps most significantly, survival is worse in prostate cancer patients having experienced an SRE [40, 41].
Bone markers have been identified that allow the rate of bone turnover to be measured. Markers such as bone-specific alkaline phosphatase and osteocalcin are markers of bone formation. Markers of bone resorption include calcium and hydroxyproline. These markers lack specificity and therefore utility. Bone turnover markers specific to bone resorption include the degradation products of type 1 collagen such as N-terminal telopeptide (NTx), C-terminal telopeptide (CTx), pyridinium crosslinks pyridinoline (PYD), and deoxypyridinoline (DPD). Elevated levels of bone turnover markers are associated with progression of cancer, increased SRE risk and decreased survival [42].
Fragility Fractures Associated with ADT
While ADT is used in metastatic prostate cancer, it is also commonly used in men without metastases. ADT results in profound hypogonadism, which is a major risk factor for osteoporosis in men [43]. Initiation of ADT in men with non-metastatic prostate cancer is associated with loss of BMD and an increased risk of fragility fractures [44, 45]. Multiple clinical risk factors help to predict fracture risk including age, personal history of fragility fracture, family history of fragility fracture, smoking, excessive alcohol consumption, and corticosteroid use [46]. The fracture risk assessment tool (FRAX) is used to predict fracture risk by integrating clinical risk factors with or without BMD [47].
Bisphosphonates in Advanced Prostate Cancer
Bisphosphonates are structural analogues of pyrophosphate and are adsorbed onto hydroxyapatite in the extracellular matrix of bone where they inhibit osteoclast mediated bone resorption. First-generation bisphosphonates such as clodronate and etidronate are relatively low-potency antiresorptive agents. Second-generation molecules such as pamidronate and alendronate have a nitrogen-containing side chain that confers increased potency. Third-generation molecules such as ibandronate, risedronate, and zoledronic acid are the most potent antiresorptive bisphosphonates.
Clodronate was evaluated in a placebo-controlled, randomized trial in 311 patients with castrate-sensitive prostate cancer with bone metastases. Clodronate failed to achieve a statistically significant improvement in bone progression-free survival or overall survival [48]. Pamidronate has been studied in placebo-controlled trials in subjects with mCRPC. In two phase II randomized studies including 378 patients, pamidronate did not reduce the incident of SREs [49].
Zoledronic acid was evaluated in a placebo-controlled phase III trial in patients with mCRPC metastatic to bone [50] (Table 10.1). In the 039 trial, 643 patients were randomized to zoledronic acid or placebo IV every 3 weeks. The trial initially included cohorts of 4 and 8 mg of zoledronic acid. However, given evidence of renal toxicity, the 8 mg cohort was reassigned to 4 mg and infusion time was increased from 5 to 15 min. Treatment with zoledronic acid lead to a statistically significant decrease in the incidence of SREs at 15 months (33.2 vs. 44.2 %, p = 0.021). The time to first SRE was 488 days for the 4 mg zoledronic acid group and 321 days for the placebo group (p = 0.009) [39]. A modest effect on pain was noted with a statistically significant advantage in pain for the 8/4 mg cohort versus placebo.
Table 10.1
Key trials of zoledronic acid and denosumab in advanced prostate cancer
Study | N | Population | Arm 1 | Arm 2 | Treatment duration (months) | Primary outcome | Regulatory approval |
|---|---|---|---|---|---|---|---|
Zoledronic acid 039 [50] | 643 | mCRPC | Zoledronic acid IV Q3 weeks | Placebo | 15a | Proportion of subjects with an SRE at 15 months 33.2 % (arm 1) vs. 44.2 % (p = 0.021) | Yes |
Denosumab HALT 138 [45] | 1,468 | Non-metastatic prostate cancer on ADT | Denosumab 60 mg SC Q6 months | Placebo | 36 | BMD at LS at 24 months +5.6 (arm 1) vs. −1.0 % (p < 0.001) | Yes |
Denosumab 147 [55] | 1,432 | Non-metastatic CRPC | Denosumab 120 mg SC Q4 weeks | Placebo | 20.2 (arm 1) 19.0 (arm 2) | Bone-metastasis-free survival 29.5 (arm 1) vs. 25.2 months (p = 0.028) | No |
Denosumab 103 [56] | 1,904 | mCRPC | Denosumab 120 mg SC + Placebo IV Q4 weeks | Zoledronic acid 4 mg IV + Placebo SC Q4 weeks | 12.2 (arm 1) 11.2 (arm 2) | Time to first on-study SRE 20.7 (arm 1) vs. 17.1 months (p = 0.0002)b | Yes |
Adverse events more common with zoledronic acid included fatigue, anemia, fever, myalgia, and lower extremity edema. Severe grade hypocalcemia was uncommon with zoledronic acid (2 %). After the trial amendment, rates of renal deterioration were similar with zoledronic acid compared to placebo (15.2 vs. 11.5 %). Though ONJ was not reported, an association between bisphosphonate use and ONJ was not reported until after publication of the study results [51].
Zoledronic acid has also been evaluated in two randomized studies to evaluate the prevention of bone metastases. One trial in men with non-metastatic CRPC and rising PSA with no bone metastases was closed prematurely to accrual after an interim analysis revealed a lower than expected event rate [52]. The ZEUS trial included 1,433 subjects with high-risk prostate cancer that were randomized to zoledronic acid every 3 months for 4 years or observation. Results of ZEUS were presented at the European Association of Urology 28th Annual Congress in 2013. There was no difference in rates of bone metastases between the two groups.
Bisphosphonates have been shown to improve BMD relative to controls in patients with non-metastatic prostate cancer treated with ADT [53, 54]. However, no trial of a bisphosphonate in this setting has been powered to detect a difference in fracture risk.
Zoledronic acid has received regulatory approval for use in prostate cancer. In the USA, approval stipulates that prostate cancer patients with bone metastases must have failed at least one hormonal agent. Both zoledronic acid and pamidronate have received regulatory approval for the treatment of hypercalcemia of malignancy.
Denosumab in Advanced Prostate Cancer
Denosumab is a monoclonal antibody targeting RANKL. As previously stated, RANK–RANKL signaling promotes osteoclastogenesis as well as osteoclast survival and function. Denosumab has been evaluated in multiple settings in advanced prostate cancer and these studies will be reviewed here.
In the Hormone Ablation Bone Loss Trial (HALT 138), 1,468 men with non-metastatic prostate cancer treated with ADT were randomized to receive denosumab 60 mg or placebo SC every 6 months [45] (Table 10.1). This trial included patients at high-risk for fragility fractures based on the following criteria; age ≥ 70, decreased BMD, or a history of a fragility fracture. Patients were stratified by age (<70 or ≥70) and duration of ADT (≤6 or >6 months). The primary outcome measure was BMD at the lumbar spine at 24 months. Secondary endpoints included the incidence of new vertebral fractures at 36 months, fracture at any site, and time to first clinical fracture.
BMD in the lumbar spine increased by 5.6 % at 24 months with denosumab and decreased by 1.0 % with placebo (p < 0.001). New vertebral fractures were more common with placebo (3.9 vs. 1.5 %; p = 0.006). The incidence of any fracture favored denosumab but did not reach statistical significance (7.2 vs. 5.2 %; p = 0.10). Fractures occurring at more than one site were more common with placebo (2.5 vs. 0.7 %; p = 0.006). There was no difference in time to first clinical fracture between the two groups. Bone turnover markers decreased with denosumab relative to placebo. Cataracts were more common with denosumab. No additional adverse events were clearly more common with denosumab. An increased incidence of cataracts has not been seen in any other studies of denosumab. Denosumab has received regulatory approval for use in men at high-risk for fracture with non-metastatic prostate cancer treated with ADT.
Denosumab has been evaluated to delay bone metastases in patients with non-metastatic CRPC [55] (Table 10.1). This trial included 1,432 patients at high-risk for developing metastatic disease as defined by a PSA of ≥8.0 μg/L or a PSA doubling time of ≤10 months. Subjects were stratified by PSA criteria (one or both) and by previous chemotherapy (yes or no). Participants were randomized to denosumab 120 mg or placebo SC every 4 weeks. Bone scans were performed every 4 months and skeletal surveys were done on an annual basis. The primary efficacy measure was bone-metastasis-free survival and favored denosumab (29.5 vs. 25.2 months; p = 0.028). Denosumab treatment also significantly delayed the time to first bone metastasis and decreased the incidence of symptomatic bone metastases. However, there was no improvement in progression-free or overall survival with denosumab.
The incidence of ONJ was 5 % with denosumab. Severe grade hypocalcemia occurred in 1.3 % of patients treated with denosumab. Based on the lack of survival difference and the high incidence of adverse events, most notably ONJ, the United States Food and Drug Administration rejected approval of denosumab to delay bone metastases in patients with non-metastatic CRPC.
Zoledronic Acid Versus Denosumab in mCRPC
Zoledronic acid has been compared to denosumab for the prevention of SREs in men with CRPC and bone metastases [56] (Table 10.1). In this randomized, double-blinded study, 1,904 subjects were randomized to denosumab 120 mg SC and placebo IV every 4 weeks or to zoledronic acid at 4 mg IV and placebo SC every 4 weeks. Stratification factors included previous SRE (yes or no), PSA (<10 or ≥10 mg/ml), and chemotherapy for prostate cancer in 6 weeks prior to randomization (yes or no). The primary efficacy endpoint was time to first on-study SRE and favored denosumab (20.7 vs. 17.1; p = 0.0002 for non-inferiority and p = 0.008 for superiority). Denosumab was a more potent suppressor of serum bone-specific alkaline phosphatase and of uNTx.
Denosumab caused more hypocalcemia (13 vs. 6 %; p < 0.0001). The incidence of ONJ was 2.3 % with denosumab and 1.3 % with zoledronic acid (p = 0.09). No increased incidence of adverse events potentially related to renal impairment was seen with zoledronic acid. Acute phase reactions were more common with zoledronic acid. Denosumab has been approved for use in solid tumors with bone metastases. Unlike the approval of zoledronic acid, approval for denosumab in this population does not stipulate castration-resistance.
Radiopharmaceuticals
Radiopharmaceuticals have several potential advantages relative to the use of external beam radiotherapy in the treatment of bone metastases. These agents are deposited in bone and preferentially at sites of active bone turnover such as areas of bone metastases with a relative sparing of uninvolved bone marrow [57]. As such, all bone metastases in an individual patient are exposed to these agents with minimal exposure to uninvolved tissues.
Beta-emitting radiopharmaceuticals include strontium-89 and samarium-153 conjugated to lexidronam (EDTMP). While beta-emitters have been shown to palliate pain, no survival advantage has been demonstrated [58]. The range of beta particles is on the order of millimeters [59], which suggests that bone marrow elements adjacent to metastases are likely to receive some radiation. Thus, despite the targeting of these radiopharmaceuticals to sites of bone metastases, the major toxicity is myelosuppression. Strontium-89 is associated with an approximately 20–30 % decrease in platelet and leukocyte count with a nadir approximately 6 weeks following treatment, with severe grade hematologic adverse events being uncommon in appropriately selected patients [57, 60]. Samarium is associated with a similar degree of myelosuppression [61, 62].
More recently, the alpha-emitting radiopharmaceutical radium-223 dichloride (alpharadin) has been developed for use in mCRPC. Compared to beta-emitters, alpha-emitting radioisotopes have a shorter range of tissue penetration of <100 μm. This corresponds to only several cell diameters resulting in less exposure to uninvolved bone marrow. Alpha-emitters also have a higher linear-energy transfer conferring them with a greater ability to cause DNA damage in prostate cancer cells. DNA and thus kill cancer cells.
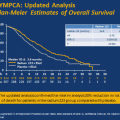
In the Alpharadin in Symptomatic Prostate Cancer Patients (ALSYMPCA) study 921 patients with CRPC and bone metastases were randomized 2:1 to receive six monthly injections of intravenous radium-223 or placebo [63]. Subjects were required to have two or more bone metastases. Subjects were required to have symptoms attributable to bone metastases as determined by the regular use of analgesics or treatment with external radiotherapy for bone metastases in 12 weeks prior to randomization. Participants were not required to have received previous chemotherapy. Stratification factors included previous treatment with docetaxel (yes or no), alkaline phosphatase level (<220 or ≥220 units/L), and current bisphosphonate use (yes or no). Exclusion criteria included a history of visceral metastatic disease or lymph node metastases of greater than 3 cm in greatest dimension. The primary outcome measure was overall survival. Secondary endpoints included the time to first symptomatic skeletal event. Median overall survival was superior in subjects treated with radium-223 [14.9 vs. 11.3 months; hazard ratio (HR), 0.70; 95 % confidence interval (CI) 0.58–0.83; p < 0.001]. The median time to first symptomatic SRE was also longer with radium-223 (15.6 vs. 9.8 months; HR, 0.66; 95 % CI 0.52–0.83; p < 0.001) (Table 10.2). Other secondary endpoints such as PSA and alkaline phosphatase response also favored radium-223. Radium-223 was well tolerated with no clear increase in severe grade hematologic or non-hematologic adverse events relative to placebo. Radium-223 has received regulatory approval for use in CRPC with symptomatic bone metastases and no visceral metastatic disease.
Table 10.2
Impact of disease modifying agents on skeletal morbidity
Study | N | Population | Agent | Overall survival | SREs |
|---|---|---|---|---|---|
COU-AA-301 [64] | 1,195 | mCRPC (prior chemo) | Abiraterone with prednisone | 14.8 vs. 10.9 months; HR, 0.65; p < 0.001 | 9.9 vs. 4.9 monthsa |
AFFIRM [65] | 1,199 | mCRPC (prior chemo) | Enzalutamide | 18.4 vs. 13.6 months; HR, 0.63; p < 0.001 | 16.7 vs. 13.3 months; HR, 0.69; p < 0.001b |
ALSYMPCA [63] | 921 | mCRPC (with bone pain) | Radium-223 | 14.9 vs. 11.3 months; HR, 0.70; p < 0.001 | 15.6 vs. 9.8 months; HR, 0.66; p < 0.001c |
Other Agents that Reduce SREs
A number of recently approved agents in the treatment of mCRPC have been demonstrated to delay SREs. In the COU-AA-301 trial, 1,195 patients with mCRPC previously treated with chemotherapy were randomized 2:1 to treatment with abiraterone plus prednisone or placebo plus prednisone [64]. Overall survival, the primary endpoint, favored abiraterone (14.8 vs.10.9 months; p < 0.001). An exploratory endpoint, the time at which 25 % of participants experienced a skeletal event favored the abiraterone group (9.9 vs. 4.9 months) (Table 10.2).
In the AFFIRM trial, enzalutamide was evaluated in patients with mCRPC who had received previous chemotherapy [65]. In this trial, 1,199 participants were randomized 2:1 to enzalutamide or placebo. Median overall survival was longer with enzalutamide (18.4 vs. 13.6 months; p < 0.001). The time to first SRE was evaluated as a secondary endpoint and favored enzalutamide (16.7 vs. 13.3 months; HR, 0.69; p < 0.001) (Table 10.2).
Disease progression is a risk factor for the development of SREs [66]. Therefore, it is not surprising that active agents that have a survival advantage are also associated with a delay in SREs. This has invited some to question if osteoclast inhibitors are as important in an era with better treatment options for mCRPC. In a post hoc analysis of data from the COU-AA-302 trial evaluating abiraterone in patients with mCRPC who were asymptomatic or mildly symptomatic and had not previously been treated with chemotherapy, the impact of treatment with a concomitant bone-targeted therapy was evaluated [67]. Bone-targeted therapy was associated with a delay in symptomatic progression, delay in decline of functional status and improved survival suggesting that bone-targeted agents provide additional benefit to abiraterone in this setting. In the TRAPEZE trial, subjects with mCRPC were treated with docetaxel and were randomized to receive zoledronic acid, strontium-89, or both agents. Zoledronic acid was associated with an improved SRE free interval (18.1 vs. 13.1 months; p = 0.008) [68]. These trials demonstrate that bone-targeted agents remain indispensable in appropriately selected patients with mCRPC.
Potential Complications of Bone-Targeted Agents
Osteonecrosis of the Jaw
Osteoclast inhibitors are associated with ONJ (Table 10.3). ONJ refers to the presence of exposed bone in the jaw that persists for at least 2 months despite appropriate therapy [69]. The first case reports of ONJ associated with bisphosphonate use surfaced in 2003 [51]. The incidence of ONJ varies between osteoclast inhibitors and with dosing of these agents. Higher rates of ONJ appear to occur with more potent osteoclast inhibitors and with higher or more frequent dosing of these drugs. In the placebo-controlled trial of zoledronic acid in mCRPC with bone metastases, no cases of ONJ were reported, but an association with these agents and ONJ had not yet been established at the time this trial was reported [50]. No cases of ONJ were reported in the trial of denosumab given at 60 mg every 6 months in patients with prostate cancer on ADT [45]. In the bone metastasis prevention trial, ONJ was reported in 5 % of patients given denosumab at 120 mg monthly [55]. In the trial comparing denosumab to zoledronic acid in mCRPC, ONJ occurred in 2.3 and 1.3 % of subjects, respectively (p = 0.09) [56].
Table 10.3




Notable adverse events reported in key trials of zoledronic acid and denosumab
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree