Fig. 14.1
Treatment delivery using portal imaging with digitally reconstructed radiograph (DRR) (a) and cone beam CT (b) to improve target visibility
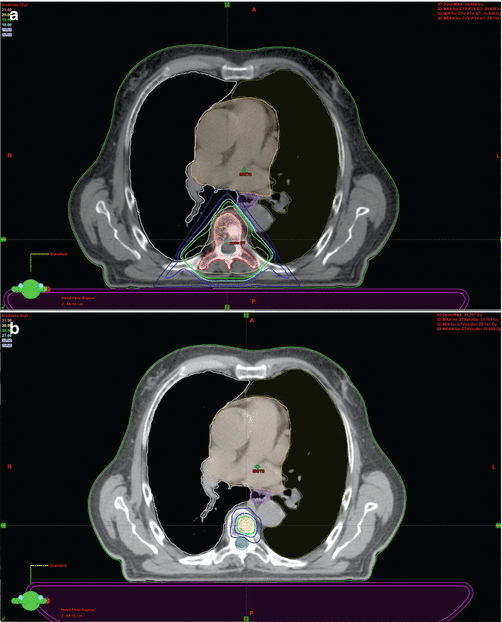
Fig. 14.2
Comparison between conventional planning with 3D-CRT (20 Gy/5 fractions) (a) and SBRT treatment with VMAT (30 Gy/3 fractions) (b) in a single vertebral metastatic localization
14.2 External Beam Radiotherapy
Prostate cancer has a tropism for bone, which is frequently the first site of metastatic dissemination [9]. More than 80 % of men with prostate cancer have a radiological evidence of bone involvement, and skeletal complications are the major causes of comorbidity in these patients. According to the natural history of the disease, bone metastases are in general initially asymptomatic. Approximately 40 % of patients will be then affected by bone pain, 20 % will experience a pathologic fracture, and 5 % will develop a spinal cord compression [10]. EBRT has a crucial role in the treatment of bone metastases, particularly in symptomatic states of the disease. The primary goals of EBRT in these scenarios are as follows: palliation of pain, prevention of skeletal events (pathological fractures and spinal cord compressions), and reversion of neurological impairment due to compression of spinal cord or spinal nerves.
Studies conducted in these settings were essentially focused on the definition of optimal prescription of dose, on the effect of different dose schedules comparing both radiotherapy in single-fraction and multiple-fraction regimens, on the duration of pain relief, on the necessity and safety of re-treatment, and on management of metastatic spinal cord compression.
The association of zoledronic acid to androgen deprivation in patients with more aggressive diseases could have a potential role in the prevention of metastatic bone progression in the context of an integrate multimodal radiation treatment [11]. Preliminary results from the TROG 03.04 RADAR trial showed an advantage in biochemical control in patients with Gleason score 8–10 undergoing radical radiotherapy and long-term androgen deprivation therapy plus zoledronic acid [11]. This topic opens up a future perspective in the radiotherapy combinations and will not be further discussed in this chapter.
14.2.1 Radiation Dose and Fractionation
Radiation schedules in EBRT have been widely evaluated in several randomized and nonrandomized studies, all comparing single fraction with different multifractionated regimens.
The Bone Pain Trial Working Party published one of the three largest randomized trials (Table 14.1) of single versus multiple-fraction palliative EBRT [12]. Seven hundred and sixty-five patients were randomized to receive 8 Gy in one fraction vs. multifractionated regimen (20 Gy/5 fractions or 30 Gy/10 fractions). Primary endpoint was pain relief. With an overall survival of 44 % at 12 months, there were no differences between the two groups in time of pain response, pain relief, and first improvement of pain. Re-treatment rate was twofold in single-fraction group, with the same probability of pain relief after re-treatment in both groups. No differences in toxicities, spinal cord compression, or pathological fracture between single-fraction and multifractionated schedules were seen [12]. The Dutch Bone Metastasis Study had as primary goal the evaluation of efficacy of single-fraction 8 Gy compared to 4 Gy × 6 fractions [13]. One thousand one hundred seventy-one patients were randomized to the two treatment schedules. Median survival was 7 months. Seventy-one percent of patients had pain relief at some time during the first year, and the two treatment schedules were equivalent in terms of palliation. Re-treatment rate was 16 % and was four times greater for single fraction; an acceptable pain relief was achieved. More pathological fractures were observed in single-fraction group, although the absolute percentage was low (3 %). No differences in treatment-related toxicities were found [13]. A subsequent analysis of the same study excluding re-treated patients confirmed that single fraction of 8 Gy was equally effective as 30 Gy in ten fractions. It was pointed out that response to initial treatment depended on the primary site of disease. Patients with prostate cancer had the lowest success rate, while patients with breast cancer had the highest percentages of response. Furthermore, re-treatment was found to be the most beneficial in patients treated with single fraction than in patients treated with multiple fractions [14]. Hartsell et al. published the results of a phase III trial, conducted by the Radiation Therapy Oncology Group (RTOG) and the North Central Cancer Treatment Group (NCCTG), including patients with breast and prostate cancer bone metastases [15]. Eight hundred ninety-eight patients were randomly assigned to receive 8 Gy in one fraction or 30 Gy in ten fractions. Complete or partial improvement of pain was observed in 66 % of patients after 3 months from randomization. No differences in response rate were found between the two groups. Re-treatment rate was twice in 8 Gy arm compared to 30 Gy arm (18 % vs 9 %). Grade 2–4 acute toxicity was more frequent in multifractionated group than in single-fraction group (17 % vs. 10 %). Late toxicity was rare [15].
Table 14.1
Summary of studies on SBRT in bone metastases from prostate cancer
Author | Year | Number of patients | Dose/fractionation | Primary disease | Overall response rate | Re-treatment rate |
|---|---|---|---|---|---|---|
Bone Pain Trial Working Party [12] | 1999 | 761 | 8 Gy/1 (n = 383) 20 Gy/5 or 30 Gy/10 (n = 378) | Breast (36 %) Prostate (33 %) Lung (13 %) Other (16 %) Unknown (2 %) | 78 % (no differences between two groups) | 23 % (8 Gy/1) 10 % (20 Gy/5–30 Gy/10) |
Steenland et al. (Dutch Bone Metastasis Study) [13] | 1999 | 1171 | 8 Gy/1 (n = 585) 4 Gy/6 (n = 586) | Breast (39 %) Prostate (23 %) Lung (25 %) Other locations (13 %) | 71 % (No differences between two groups) | 25 % (8 Gy/1) 7 % (4 Gy/6) |
Hartsell et al. [14] | 2005 | 898 | 8 Gy/1 (n = 455) 30 Gy/10 (n = 443) | N.R. | 66 % (no differences between two groups) | 18 % (8 Gy/1) 9 % (30 Gy/10) |
All published randomized trials were analyzed in a series of comprehensive meta-analysis [16–18], the most recent including 5263 patients enrolled in 25 randomized controlled trial [19]. Results showed that single- and multiple-fraction regimens provided similar complete and overall response rates. Pathological fractures rate was equal in both groups, while the reduction of spinal cord compression trended in favor of multiple fractions, without statistical significativity. The re-treatment rate was 2.6-fold greater in single-fraction arm. No differences in acute toxicities were seen between the two groups. A subanalysis of patients with neuropathic pain enrolled in a randomized trial published by Roos et al. was also performed, showing that single fraction of 8 Gy was less effective than a multifractionated regimen of 20 Gy in five fractions, without reaching statistical significance [19, 20].
The optimal dose of single fraction able to achieve pain relief without significant side effects is currently unknown. Dennis et al. in a systematic review analyzed single-fraction arms in 24 randomized trials including 3233 patients [21]. The most widely prescribed dose was 8 Gy (84 % of the patients, n = 2717). The other delivered doses were 4 Gy (n = 246), 5 Gy (n = 14), 6 Gy (n = 108), and 10 Gy (n = 134). Only three of considered trials directly compared single-fraction arms with an advantage in pain response of 8 Gy vs. 4 Gy and a non-superior response of 8 Gy vs. 6 Gy. Authors concluded that defining the single fraction of 8 Gy as the optimal dose is still an open question, and future clinical trials should directly compare doses and radiation techniques [21].
Despite the results of randomized studies that showed equal efficacy in pain relief between single fraction and multiple fractions in uncomplicated bone metastases, a reluctance to employing single fraction was highlighted in the radiation oncology community [22]. A survey to assess pattern of practice on 962 members of three radiation oncology professional organizations (ASTRO, American Society for Radiation Oncology; CARO, Canadian Association of Radiation Oncology; Royal Australian and New Zealand College of Radiologist) was conducted. Five hypothetical cases of bone metastases from different primary cancers (breast, lung, or prostate) were proposed. The responses of surveyed physicians included 101 different treatment schedules (range, 3 Gy/1 fraction to 60 Gy/2 fractions; median dose 30 Gy/10 fractions). Most radiation oncologist continued to prescribe multifractionated schedules. A prescription trend versus single-fraction radiotherapy was seen as a function of country of training (Canada and Europe vs. the United States) and location of practice (university or academic centers vs. private institutions) [22]. A recent review published by Popovic et al. [23] analyzed results derived from international surveys administered to radiation oncologist examining actual prescription practice for painful metastases. Considering the most recent patient data, there was not an increasing trend of prescription of single-fraction dose in function of treatment year in uncomplicated bone metastases. Geographical differences were found; Canadian physicians were more readily prescribing single fraction than physicians from the United Kingdom or Norway. Authors concluded that, despite clinical evidence, there was a global reluctance in the prescription of single fraction. Many factors influenced this trend and were related to radiation oncologist preferences, patients, radiotherapy centers, and personal beliefs [23].
14.2.2 Reirradiation
In patients with bone metastases, radiotherapy has a role in palliation of pain, even in a re-treatment setting. Re-treatment rates are about 8 % in patients previously irradiated with multifractionated radiotherapy and 20 % in patients treated with single fraction [19].
New and more sophisticated therapies and improvements in supportive care resulted in increased survival of patients, leading to the need to receive further radiation treatments [24].
Reirradiation can be considered in three different settings: (1) no pain relief or progression of pain after a first course of radiation, (2) partial response to initial treatment and the need to achieving further pain reduction, and (3) partial or complete response to initial radiotherapy and subsequent pain progression [25].
Available studies on reirradiation were analyzed in a systematic review conducted by Wong et al. [26], updating a previously review of Huisman et al. [27]. Fifteen articles with an evaluable population of 645 patients were considered. Overall response rate to reirradiation was 68 %, with 20 % of patients experiencing a complete response to the treatment. Patients who had a previous complete response to radiotherapy were more likely to achieve a complete and more durable pain response upon re-treatment than patients with initial partial response. Toxicities were not consistently reported; however, no grade 3 or 4 toxicity, pathological fractures, and spinal cord compression were recorded [26].
A consensus on when performing reirradiation after a first course of radiotherapy hasn’t been reached yet [28]; in a survey conducted by the International Bone Consensus Working Party, the majority of radiation oncologist considered for reirradiation a time period beginning 4 weeks after completion of the initial treatment course [28, 29].
Available data are not conclusive to define optimal dose and fractionation when a reirradiation is needed. The ASTRO evidence-based guidelines on palliative radiotherapy for bone metastases recommend to treat patients within the already available clinical data on doses and fractionation schedules [30]. Chow et al. published the results of a randomized trial, aiming to compare two different dose fractionation schedules (8 Gy in one fraction versus 20 Gy in multiple fractions) in patients undergoing a repeated radiotherapy. Four hundred and twenty-five patients were assigned to each treatment group. Primary endpoint was overall pain response at 2 months (complete and partial response were included). No differences were seen between the two groups in pain relief and in freedom of pain progression. No cases of radiation myelitis were reported. Authors concluded that repeated radiation therapy was effective, irrespective to the adopted treatment schedule. Forty-eight percent of all patients had a reduction of pain in re-treated sites, and 68 % of patients reported an improved quality-of-life pain score. Eight Gy seemed to be non-inferior to multifractionated schedule, but it wasn’t excluded that a small proportion of patients could benefit more from multiple fractions [31]. Available clinical data were also analyzed with the aim of building a prognostic model, considering different risk groups to predict survival in patients requiring reirradiation for painful bone metastases. Authors recommend the use of 8 Gy in single fraction in the worst group identified in their survival model (low Karnofsky performance status score and more aggressive primary cancer site, such as the lung) [32].
14.2.3 EBRT in Management of Spinal Cord Compression or Pathological Fractures
Metastatic spinal cord compression (MSCC) is an oncologic emergency and may cause paralysis, sensory loss, and sphincter incontinence, if left untreated [33, 34]. EBRT is generally the treatment of choice for MSCC. Two randomized phase III multicenter Italian trials were conducted with the aim of defining the most effective radiation schedule in patients with MSCC and short life expectancy [35, 36]. The first compared a short-course radiotherapy (8 Gy × 2 days) with a split-course regimen (5 Gy × 3, 3 Gy × 5). Two hundred and seventy-six patients were randomized to the two different schedules. Pain relief was achieved in 56.9 % of patients, and no significant difference in response rate or survival was found. Toxicity was similar and acceptable in both radiation schemes. Authors concluded that a short-course regimen could be the best and more convenient choice for patients with MSCC and short life expectancy [35]. On the premise of the first trial, the second randomized study started with the aim to define whether, in the same category of patients, 8 Gy in single fraction was effective as 8 Gy in two fractions. Three hundred and three patients were enrolled. Results showed no differences in response rates for back pain, motor function, and sphincter control between the two treatment arms. Pain relief was achieved in 53 % of patients. Tolerance to both regimens was good and, after a median follow-up of 31 months, no myelopathy was registered [36]. Among 579 patients enrolled in the two randomized trials, Maranzano et al. analyzed the outcomes of patients undergoing reirradiation for local relapse. Only 50 % of the 24 relapsed patients were re-treated, and different re-treatment schedules were used. Patient walking capacity before reirradiation was a predictor of functional outcome, and no case of myelopathy was recorded [37].
Rades et al. published the results of a prospective study comparing local control of short-course (8 Gy x1 or 5 Gy × 4) and long-course radiotherapy (3 Gy × 10 or 2.5 Gy × 15 or 2 Gy × 20) in patients with MSCC and long life expectancy [38]. Primary endpoint was local control. Motor function and survival were analyzed as secondary focuses of interest. Results showed, on 265 patients included in the study, a significantly better local control after long-course radiotherapy than after short-course radiotherapy (81 % vs. 61 %, p = 0.0005). Radiation schedule had no significant impact on functional outcomes or overall survival. Acute toxicity was moderate and no cases of myelopathy were recorded. Authors concluded that patients with a relatively favorable survival prognosis might have higher risk for local relapse and benefit from long-course radiotherapy, while patients with a poor expected survival appeared adequately treated with short-course radiotherapy [38]. EBRT can also be combined with decompressive surgical resection in selected patients. Patchell et al. [39] analyzed functional outcomes in 101 patients with MSCC randomized to receive decompressive surgery followed by radiotherapy or radiotherapy alone. Surgical group outcome was significantly better than radiotherapy group in ability to walk after treatment (84 % vs. 57 %, respectively). Surgery plus radiotherapy allowed to most patients to remain ambulatory since dead and reduced the use of corticosteroids and opioid. Combined treatment also had an impact on overall survival. The trial was stopped early because of the superiority of surgical treatment outcomes. However, this approach should be proposed in selected patients; very radiosensitive tumors, multiple areas of spinal cord compression, or total paraplegia for more than 48 h were exclusion criteria from the trial [39]. A subsequent analysis of this study showed that age was a relevant variable in predicting ambulatory preservation and survival. In patients ≥65 years, there were no difference in outcome between EBRT alone and surgery followed by EBRT [40].
The role of postoperative radiotherapy in patients with metastatic pathological fractures underwent to orthopedic stabilization was reported in a retrospective study published by Towsend et al [41]. Thirty-five of a total of 64 patients had combined treatment (surgery plus radiation). Functional status was significantly better in patients treated with surgery and radiation than with surgery alone (53 % vs. 11.5 %, respectively), suggesting the benefit of radiotherapy in this setting [41]. In a more recent retrospective study, Wolanczyk et al. reported the outcome of 72 patients undergoing surgical stabilization for complete or impending pathologic fractures and subsequently treated with EBRT with or without zoledronic acid (43 % and 44 % of patients, respectively) or zoledronic acid alone (13 % of patients) [42]. Median overall survival time was 14 months, and median prescribed dose was 30 Gy (30–40 Gy/2–3 Gy per fraction). Local progression, pain progression, and need for re-treatment (surgery or EBRT) were analyzed. Local tumor progression was 7 % and 9 %, respectively, in irradiated patients with or without zoledronic acid and 44 % in patients treated with zoledronic acid alone (p = 0.02). Local pain progression was 16 %, 19 %, and 44 % in the same groups, respectively (p = 0.011). No difference was seen in re-treatment rate between the different groups. Authors concluded confirming the efficacy and the need of postoperative EBRT after orthopedic stabilization for metastatic bone disease [42].
14.3 Hemibody Irradiation
In the past years, hemibody irradiation (HBI) was often used for the treatment of widespread, symptomatic, metastatic bone disease from different types of cancers, prostate included.
Since 1970s, this technique evolved for palliation of uncontrolled pain in patients with a massive bone involvement, with the advantage of treating many sites fast and simultaneously.
The response rates were similar to those of the irradiation with local fields, but pain relief was more rapid, occurring often after 48 h from irradiation [43–47].
HBI can be delivered with different field sizes and different patient positions. Three different types of field arrangements were more frequently described: upper half-body irradiation (UBHI) from the top of the head to the level of the iliac crest (L4-L5 interface), lower half-body irradiation (LHBI) from iliac crest to the ankles, and midportion-body irradiation (MBI) from the top of the diaphragm to the bottom of the obturator foramen [48].
Consensually with the development of this technique, the Radiation Therapy Oncology Group began a series of studies in order to evaluate the efficacy and safety of this treatment. RTOG 78-10 showed that a single fraction of HBI was effective, with pain relief in 73 % of patients, and identified as safest doses 6 Gy for UBHI and 8 Gy for LHBI and MBI. Increased toxicity was seen with higher doses without clinical benefit [46, 49].
RTOG 82-06 explored the possibility that HBI added to irradiation with local fields had an effect on occult disease with a delay of appearance of new metastases and a decrease of the frequency of further treatments. Patients were randomized to receive the standard palliative treatment (3 Gy × 10 fractions) with or without HBI. The results demonstrated an advantage for the HBI group in time-to-disease progression (35 % for local + HBI vs. 46 % in local only) and time-to-new disease at 1 year (50 % for local + HBI vs. 68 % in local only), without having an impact on overall survival. The maximum benefit was observed in breast and prostate cancer, but an increased toxicity was recorded with the use of HBI [50].
RTOG 88-22 protocol started with the aim to evaluate fractionated HBI; the results showed a modest improvement in reduction of local failure in the fractionated schedule, with a maximum tolerated dose of 17.5 Gy (2.5 Gy per fraction). It was concluded that single high-dose HBI was as effective as fractionated HBI. The major dose limiting toxicity was hematological (thromboleukopenia) [51].
The main problem with respect to HBI, even when it’s carried out in single fraction, is the need of hospitalization or close monitoring due to the necessity of premedication with steroids, hydration, and antiemetics administration to prevent acute toxicity, particularly in the treatment of upper body. The majority of treated patients experiences gastrointestinal toxicity such as nausea and vomiting, particularly in the first hours after the irradiation, and diarrhea lasting 3–7 days. All symptoms are transient. Hematological toxicity has a peak at 2 weeks but disappears after 4–6 weeks from the HBI exposure and rarely needs blood transfusion. Radiation pneumonitis is rare if lung dose is lower than 7 Gy [43, 46, 47].
Modified fields were used over the years, with the aim of reducing toxicity. Bashir et al reported a 10-year experience of palliative treatments with modified HBI for metastatic carcinoma of the prostate, excluding the skull and the lower leg from treatment fields; the results showed a successful outcome with pain response and reduction of analgesic intake. The treatment was easier, with a simplified setup, and patients were protected from unnecessary distressing toxicities such as alopecia, dry mouth, and parotitis [52]. A randomized phase III trial of the International Atomic Energy Agency (IAEA) aimed to find the most effective and efficient method to deliver HBI. Three different fractionation schedules were used: 15 Gy in five fractions over 5 days, 8 Gy in two fractions over 1 day, and 12 Gy in four fractions over 2 days. Pain relief was seen in 91 % of patients; toxicity was acceptable: moderate in 50 % of the patients, absent in 41 %, and severe only in 12 %. The study highlighted that all primary tumors responded to all prescribed schedules with the exception of prostate cancer, for which 15 Gy over 5 days was the most effective schedule [53].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree