Syndrome
Pain
Cranial nerve involved
Orbital
Retro-supraorbital
II visual loss
III, IV, VI diplopia
V frontal sensory loss
Cavernous sinus
Supraorbital frontal
III, IV, VI diplopia
V sensory loss
Middle cranial fossa
Trigeminal pain, paroxysmal
headache
VII, V
Jugular foramen
Mastoid, neck, shoulder
IX, X dysphagia
XI trapezoid sternocleidomastoid
Weakness
XII tongue deviation
Occipital condyle
Unilateral nuchal radiate behind the eye
Tenderness of the occipital junction
XII tongue
19.2.2 Pathophysiology of Bone Cancer Pain
The exact mechanism by which tumor in bone produces pain is not completely understood; various postulated mechanisms of bone pain include the role of prostaglandins, local change in bone metabolism and blood flow, and the stimulation of nerve endings by increased intraosseous pressure, cytokines, and locally released neurotransmitters.
Tumors may secrete proteases that cause bone cell lysis, and yet osteoclastic activity is required to break down the mineralized bone matrix; also, tumor cells secrete a number of factors that enhance osteoclastic activity. As the tumor expands outward from the marrow space, it may cause increased intraosseous pressure, particularly if the growth is rapid. This may activate mechanoreceptive nociceptors in bone and stretch the highly innervated periosteum. Edema and inflammation may also contribute to pain, via both increased pressure and secreted mediators, which, in turn, activate pain receptors. Cancer-induced bone pain is a specific pain state with overlapping but distinct features of both inflammatory and specific central and peripheral hyperexcitability mechanism [26]. Cancer cells in the body microenvironment stimulate local inflammatory mediators and create a highly acidic environment, which sensitizes peripheral nerve endings within the bone marrow and bone matrix and thus can explain how resulting pain is associated with a hyperexcitability state within the spinal cord. Peripheral and central hyperexcitability may explain why patients experience constant pain, with severe pain episodes highly sensitive to movement and other innocuous stimuli or initiated by unknown spontaneous mechanism.
Extensive bone disease can also lead to fractures and compression of adjacent nerves, vascular structures, and soft tissue.
19.2.3 Nociceptive and Neuropathic Pain
The participation of somatic bone afferents to the pathophysiology of pain due to bone metastases via the mechanisms briefly summarized above should be regarded as a source of somatic nociceptive pain according to a traditional clinical–pathological classification. The clinical impact of specific mechanisms highlighted in animal experimental models of cancer bone invasion is at the moment unknown. However, patients with pain due to bone metastases can have pain also due to lesions of nerve roots, peripheral nerves of plexus produced by the progression of their bone lesions. These lesions can cause also neuropathic pain. The diagnosis of neuropathic pain requires specific expertise and should be reserved to cases presenting with a neurological lesion associated with sensory and pain related objective symptoms and signs [27]. In all cases, the characteristics of pain should be evaluated in time to identify eventual changes in the pain mechanism as already suggested although bone metastases can cause per se somatic pain for long time periods; the progression to invade other tissue – soft tissue, viscera, and neurological structures – should always be considered.
19.3 Pain Assessment
The initial assessment of pain should include a detailed history, including the location, intensity, frequency, temporal pattern, and specific characteristics of the pain. Knowledge of factors that aggravate and alleviate the pain is also critical to an effective treatment plan. Accurate physical and neurological examination follows a good pain history.
A thorough assessment of pain must also include psychosocial components, such as the patient’s attitude toward his diagnosis and treatment, mechanisms for coping with pain and stress, psychological responses to pain (such as anxiety and depression), and attitude regarding controlled substances. These factors often play a role in both the patient’s experience of pain and his response to treatment. In addition, information regarding the patient’s support system and insurance plans often is crucial to the success of treatment.
To establish the presence of pain, pain severity, and pain relief, consideration must be given to the specific patient population, using instruments with defined psychometric properties.
The literature suggests that clinicians often underreport patients’ pain intensity in comparison to the patients’ own report of their pain. Therefore, an unfiltered representation of the patient’s experience, as measured using a patient-reported outcome, is preferred [28].
Pain intensity must be measured and recorded to help in monitoring therapy results and improve communication with patients on pain by introduction of simple scale measure. Different pain measurement tools have been validated for cancer pain. They can be divided in two main categories: intensity scale and multidimensional questionnaires. Intensity scales are visual analogue scale (VAS), numerical rating scale (NRS), and verbal rating scale (VRS) [29].
NRS seem to have common meaning across cultures while keeping some desirable psychometric properties if compared with VRS [30]. In clinical practice, the pain intensity alone may not be sufficient to evaluate treatment efficacy, and additional clinical information can suggest how much a given pain level bothers the patients, which is the level of pain that the patient considers tolerable, what corresponds to satisfactory pain relief, how the pain interferes with quality of life, and where would the patient place himself along the trade-off between pain relief and side effects.
Several instruments are available for multidimensional evaluation such as the Brief Pain inventory [31], the McGill Pain questionnaire [32], and the Memorial Pain Assessment Card [33]. All of them are valid and reliable and important research tools.
Pain needs to be continually reassessed in the prostate cancer patient since disease progression, response to treatment, and response to pain control maneuvers all influence treatment strategies. The clinician needs to educate both patients and their families and caregivers about pain, its causes and treatments, and their active participation in pain assessment and management. Education should address pain assessment, dose titration, side effect management, and any fears and misconceptions regarding addiction and tolerance.
The steps to be considered when choosing one method for systematic patient assessment are reported in Table 19.2.
Table 19.2
The steps needed for systematic pain assessment
1. | Choose method of evaluation and frequency |
2. | Establish time referral i.e., in example average of last 24 h pain or last week pain. “Pain now” is the most reliable |
3. | Pain quality and intensity change in time and episodes of breakthrough should be evaluated aside to baseline pain |
4. | A body chart showing different pain sites should be part of the regular assessment |
5. | Average and worst pain intensities are considered important clinical variables |
6. | Pain intensity measures should be visible in patient’s chart and be part of routine evaluation in oncology setting |
19.4 Pain Syndromes in Prostate Cancer Bone Pain
Cancer pain can be described under different clinical domains, etiology, location, and pain mechanism and pain syndromes.
Cancer pain syndromes can be broadly divided into those that are acute and those that are chronic. Acute pain syndromes usually accompany diagnostic or therapeutic interventions, whereas chronic pain syndromes usually are directly related to the neoplasm itself or to an antineoplastic therapy [34].
In many cases, the presence of symptoms and signs can suggest a specific cancer pain syndrome [34]. The identification of such a syndrome may help to elucidate the etiology of the pain, direct the diagnostic evaluation, clarify the prognosis for the pain or the disease itself, and guide therapeutic intervention.
Caraceni et al. [19] described pain syndrome in a review article “as repeated cluster of symptoms and sign including pain which combined with other relevant information from the history and examination, identify a clinical entity that can be used to define that specific situation.”
In a cross-sectional study, 1095 cancer patients with pain were evaluated describing pain mechanism, pain intensity, and pain syndromes. Twenty-two of 51 pain syndrome were prevalent [19].
This survey suggests the existence of disease-related syndrome clusters. A variety of bone pain syndromes were found in prostate cancer and its evolution (Table 19.3).
Table 19.3
Bone pain syndrome in metastatic castration-resistant prostate cancer
Base of skull syndrome |
Vertebral syndromes |
Diffuse bone pain |
Due to multiple bone metastases |
Due to bone marrow infiltration/expansion |
Focal bone pain |
Long bones |
Hest wall rib pain |
Infiltration of a joint (sacroiliac joint) |
Pelvic bony lesions |
Patients with prostate cancer were also more likely to experience generalized bone pain and pelvis and long bone pain than other tumor types as shown in Table 19.4.
Table 19.4
Frequency of bone pain syndromes in 65 prostate cancer patients
Base of skull | 1.5 % |
Vertebral | 21.5 % |
Pelvis and long bones | 26.1 % |
Generalized bone pain | 40.0 % |
Chest wall | 4.6 % |
Pathological fracture | 3.0 % |
19.4.1 Vertebral Pain Syndromes
Vertebral pain syndromes are the most common cause of pain in mCRPC. The thoracic spine is affected in more than two thirds of cases, the lumbosacral spine in 20 %, and cervical spine in 10 %. Tumor usually involves the vertebral body and expands posteriorly. Multiple vertebral lesions are common. Hematogenous spread is the most common route, but tumor can invade the vertebral body from contiguous paraspinal site, pelvic nodes masses. In the assessment of the neck and back, it is necessary to differentiate local pain and referred pain, which are due to stimulation of pain sensitive structures in the spine and its joints and muscles, from radicular and spinal cord-related symptoms. This is important for making for an early diagnosis of epidural spinal cord compression (ESCC) that represents the most important complication. The association of vertebral bone with radiculopathy was described in about 10 % of cases [19].
Local bone pain has a dull, aching, deep quality and can be constant of vertebral metastatic pain, but movement and postures often aggravate it. Pain on activity should be regarded as sign of potential impending bone fracture.
Pain due to vertebral metastases especially when the invasion of the epidural space is already present can be worse when lying down and better when sitting; often pain can be referred to distant body areas from vertebral lesions, though lacking the specific clinical characteristics of radiculopathies in a pseudo-dermatomal fashion (sclerotomes).
The main complications of vertebral lesions are vertebral collapse, radiculopathies, and ESCC.
Collapse of vertebral bodies is particularly frequent in the thoracic spine. They can acutely aggravate the pain syndrome by impingement on the nerve roots and can cause skeletal deformities, implying a higher risk for ESCC or cauda equine compression. Radiculopathies can develop at any level; the pain is felt on the spine, deep in the muscles innervated by the affected root, and in the corresponding dermatome. It is aggravated by position that increases tumor compression on the root, such as lying down. The diagnosis of radiculopathies requires the association of sensory, motor, and reflex findings.
19.4.2 Bony Pain Syndromes: Long Bones, Bony Pelvis, Hip, and Shoulder
Metastases to the pelvis, hip, and femur are common and are associated with incident pain on movement and on weight bearing. There is high propensity of these lesions to fracture. Femur fractures can at time be operated on, but pelvic fractures are not usually operable and severely impact on the patient’s ability to move and walk. Shoulder joints and humeri can also be infiltrated by tumors and need to be properly diagnosed to prevent fracture.
19.4.3 Hip–Joint Syndrome
When the acetabulum or the head of the femur are metastatic sites, the characteristic pain is exacerbated by leg movement and weight bearing. The pain often radiates to the knee and thigh. Pain can also be referred only to the knee. This bony lesion can extend into the pelvis and compress the lumbosacral plexus or the sciatic nerve. Lytic lesions of the femoral neck are to be managed with extreme caution even when examining the patient. Pelvic tumor can infiltrate at the same time the lumbosacral plexus and hip bone and sometimes viscera.
19.4.4 Sacroiliac–Joint Syndromes
This syndrome is characterized by local pain at the sacroiliac joint, aggravated electively by weight bearing and manual compression on the joint. The pain can also radiate to the thigh resembling a hip lesion.
19.4.5 Bone Marrow Infiltration or Bone Marrow Expansion Syndrome
Generalized bone pain with symptoms of migrating pain, often fluctuating in intensity in close relationship with therapeutic interventions, is found with diffuse marrow infiltration by solid tumors. The pain is in the limbs, and local bone tenderness is a constant finding especially on the diaphysis of long bones.
19.4.6 Base of the Skull Syndromes
Metastatic lesions to the base of the skull are relatively common during metastatic bone diffusion in prostate cancer.
Headache of moderate to severe intensity at the site of the lesion or referred to the vertex or to the entire affected site of the head is the common pain symptoms. The association of cranial nerve involvement establishes a diagnosis. The base of skull syndromes causing pain is shown in Table 19.1.
19.5 Treatment of Cancer-Induced Bone Pain
The strategies for treatment of CIBP in prostate cancer and/or their complication are reported in Fig. 19.1.
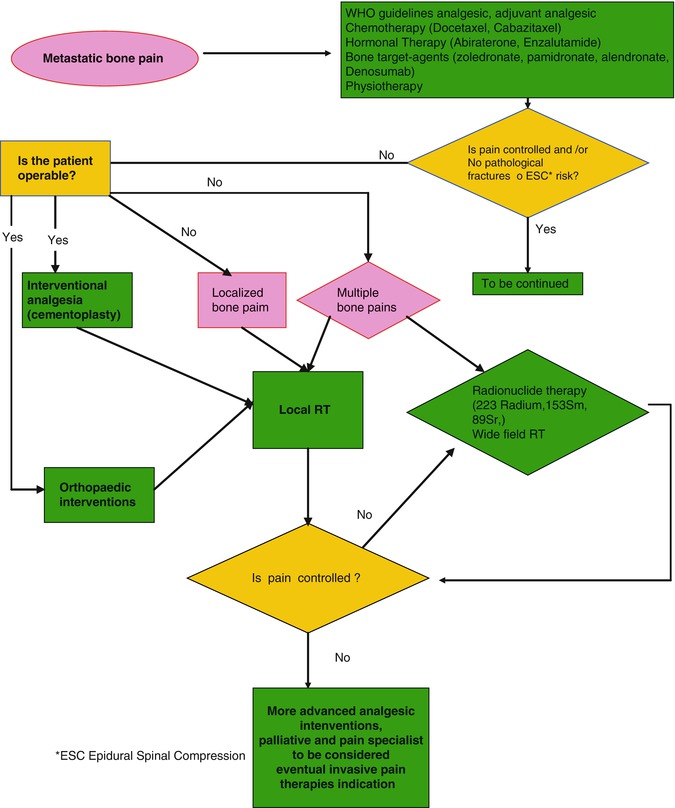

Fig. 19.1
Management of painful skeletal metastases in metastatic castration-resistant prostate cancer
Pharmacological management of CIBP involves the use of analgesic agents such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics in combination with adjuvant therapies including bisphosphonates, RANKL antibody, corticosteroids, chemotherapy agents, endocrine therapy radiotherapy, radio metabolic treatment, orthopedic surgery, and rehabilitation.
19.6 Analgesic Pharmacotherapy
Pharmacological treatment of cancer pain is largely based on the World Health Organization analgesic ladder method [35]: drugs should be given “by the clock,” rather than “on demand”; preferred way to assume drugs is by the mouth, rather than invasive routes; clinicians should avoid fixed doses of analgesics and adapt drug doses to the individual patient response; and careful management of analgesic unwanted side effects is warranted. This guideline recommends to choose the analgesic according to the pain intensity: non-opioids (paracetamol, aspirin, and nonsteroidal anti-inflammatory drugs) for mild pain management, non-opioids and second-step opioids (codeine, tramadol, and low doses of oxycodone) for moderate pain, and non-opioids and strong opioids for severe pain treatment. Additional – “adjuvants” – drugs should be added at any steps for specific pain syndromes, such as neuropathic pain and pain arising from the cancer bone invasion. A rapid-onset analgesic should be available for the management of pain exacerbations (breakthrough pain, such as pain incident to movements).
This approach is simple to be used also in nonspecialist care, inexpensive, and 80 % effective [36]. Therefore, it is the mainstay of bone cancer pain treatment. There are no specific guidelines about the management of pain induced by metastases to the bone or from prostate skeletal metastases [37].
19.7 Non-opioid Analgesic (See Also Table 19.5)
Table 19.5
Analgesic pharmacotherapy in cancer-induced bone pain
Drug class | Drug | Suggested starting dose | Comments |
|---|---|---|---|
Paracetamol | Paracetamol | 500–1000 mg, as needed or every 4–6 h | Maximum dose 4000 mg/day |
Nonsteroidal anti-inflammatory drugs Add gastroprotective drugs (as omeprazole, 20 mg tablets, or pantoprazole 20 mg tablets) in elderly and frail patients | Diclofenac | 50 mg as needed or every 6 h or 100 mg every 12 h (extended release tablets) | Maximum dose 200 mg/day |
Ketoprofen | 50 mg as needed or every 6 h or 100–200 mg once a day (extended-release capsules) | Maximum daily dose 200 mg | |
Ibuprofen | 600 mg as needed or every 6 h or 800 mg every 12 h (extended-release tablets) | Maximum dose 2400 mg/day | |
Ketorolac | 10–30 mg as needed or every 6–8 h | Maximum daily dose 120 mg. Consider only for short-period treatments | |
Opioids Add prophylactic laxatives drug, as macrogol | Codeine (in association with paracetamol 1000 mg) | 30–60 mg as needed or every 4–6 h | Maximum paracetamol daily dose 4000 mg |
Tramadol | 50 mg as needed or every 6 h or 100 mg every 12 or 24 h (extended-release tablets) up to 400 mg/day | Use lower dosage when other serotoninergic drugs are administered Consider halved dose in parenteral administration Maximal daily dose 400 mg | |
Tapentadol | 50–100 twice a day up to 500 mg/day | Titrate every 2–3 days from lower dosage. Consider tramadol or other analgesics as rescue drug for episodic pain exacerbations | |
Buprenorphine | 35–70 mcg/h, 3-day patch | Upper maximum dose 140 mcG/h. Consider other opioids, rather than buprenorphine sublingual tablets, as rescue medication | |
Morphine | 5–10 mg every 4 h immediate release formulation or 10 every 12 h (slow-release tablets) | Titrate to effect every 24 h increasing of 30–50 % of daily dose | |
Oxycodone | 5 mg (associated to paracetamol 325 mg) every 4 h 5–10 mg (alone or associated to naloxone), extended release tablets every 12 h Immediate release tablets and solutions can be used starting with 5–10 mg every 4 h | Maximum dose in naloxone-associated tablets, 160 mg/day | |
Hydromorphone | 1 mg immediate-release tablets should be given every 4 h – 8 mg once a day prolonged release formulation | Use rapid onset morphine or oxycodone–paracetamol as rescue drugs for pain exacerbations | |
Fentanyl (transdermal) | 12–100 mcg/h 3 days patch | ||
Fentanyl (intranasal or transmucosal) | 100 mcg intranasal spray or buccal tablet minimum dose (50 mcg are available for intranasal formulation) and higher dosing from 200 to 800 mcg can be used in tolerant patients | Use only for breakthrough cancer pain, in patients on oral morphine or other opioids ATC therapy of at least 60 mg morphine per day or different opioid equivalent doses Maximum 4 doses/day, with a time period of at least 4 h between doses Starting from minimum dose. Titrate to adequate dosage | |
Steroids | Prednisone | 5–25 mg once day | |
Desametasone | 0,5–4 mg once day | ||
Neuropathic pain drugs | Gabapentin | 300 mg three times a day | Maximum dose 3600 mg/day |
Pregabalin | 75 mg every 12 h | Maximum dose 600 mg/day |
Paracetamol is largely used as a first-step treatment in cancer pain, as single drug – 500–1000 mg, as needed or every 4–6 h – or combined with opioids. At doses lower than 4000 mg/day, paracetamol toxicity is not clinically relevant. However, it should be considered a weak analgesic. There are insufficient evidences of an improved analgesic efficacy adding paracetamol to a strong opioid [38, 39].
Considering the inflammatory nature of bone cancer pain [40], nonsteroidal anti-inflammatory drugs sound a rationale choice. NSAID efficacy in cancer pain is evidenced by several clinical studies [39, 41]. However, specific analyses of the bone cancer subgroup population are not available. NSAID’s gastrotoxicity is well known and increased by a concomitant steroidal therapy. When gastric risk factors are identified, a selective COX-2 NSAID, as etoricoxib, 90 mg once day, is a valid alternative. NSAIDs are contraindicated in severe renal or hepatic insufficiencies, in heart failure, and in patients with bleeding risks. Many clinicians avoid associating NSAIDs to strong opioids when managing severe cancer pain. However, when NSAID’s clinical risks are lacking, the association with opioids is advisable, since the augmented analgesic efficacy [38, 39]. Moreover, NSAID collateral effects are not increased by the opioids therapy [38].
Nonsteroidal anti-inflammatory drugs’ common choices in palliative care are diclofenac, 50–100 mg BID or TID or – fast-onset release – 50 mg on request; ibuprofen, 600–800 mg TID or as needed; ketoprofen, 50 mg TID or as needed or 200 mg slow release once day; and ketorolac, 10–30 mg, every 6 or 8 h or as needed, only for short periods of treatment. NSAIDs used in cancer pain studies have shown equivalent efficacies, when adjusted to the right dose [41]. Higher doses are more effective on pain; however, toxicity is weakly linked to NSAID’s dose and closely dependent from comorbidities and length of the treatment [41]. Intravenous, intramuscular, or subcutaneous injections or suppository route of NSAIDs is equally effective and has the same gastric toxicity of the oral forms; thus, they should be reserved only for patients with unavailability of oral route of administration. Gel or ointment NSAIDs don’t penetrate to deep tissues and are considered ineffective in cancer bone pain.
19.8 Opioids (See Also Table 19.5)
Opioids are the mainstay in the cancer pain therapy. At the right dose, opioids are very effective in cancer pain, and a skillful use could minimize the relevance of unwanted side effects. In the last years, a surge in US hospital admissions, and even deaths, provoked by opioid overdosage consequences, mainly in patients with chronic nonmalignant pain conditions, has pushed authorities to limit long-term opioid therapy eligibility and high dosages of opioid-based schedules [42]. Thus, recent guidelines suggest to refer to specialized pain clinics patients who need dosage above 80–120 mg/day morphine equivalents or who present symptoms of opioid abuse or misuse [42, 43]. Even if there are no analogous warnings in the cancer pain management, a careful conduct is warranted in long survival patients, like in the prostate cancer population.
Codeine is the prototype of the second-step opioid. Suggested schedule of treatment is 30–60 mg as needed or every 4–6 h. In the commonly used associations to paracetamol, this latter maximum dose should not exceed 4000 mg per day. Other opioid drugs with an upper limit of the dose range are the partial mu agonist buprenorphine that is most used as transdermal patches, until a maximum dosage of 140 mcg/h, and the mixed mu agonist and noradrenergic–serotoninergic analgesics tramadol, used until the dose of 400 mg/die by the mouth, and tapentadol that can be prescribed until a maximum dose limit of 500 mg/day [44]. In the last years, the strong opioid oxycodone has been combined to naloxone, in order to reduce its annoying constipating effect, as slow-release tablets, with a maximal dose of 160 mg/day. However, many clinicians are used to prescribe low doses of strong opioids (such as 5 mg of oral morphine every 4 h) also in mild cancer pain treatment, and usefulness to differentiate second-step from third-step categories of analgesics is debated [44–46].
WHO and EAPC guidelines recommend to initiate an opioid therapy using oral drugs, reserving transdermal route to non-naive patients. There are no significant differences in opioid starting therapies based on immediate release and sustained release drugs, taken at the right time [45, 47]. Experts suggest to start with low doses and carefully titrate the opioids every each day or every three days, until adequate pain control is reached [44]. A short-acting opioid (such as oral morphine immediate release) could be prescribed as a rescue analgesic, in association with long-acting opioids, to reach an adequate analgesic level in the titration period [44]. If an opioid treatment doesn’t provide an acceptable pain control, or patient experiences non-tolerable side effects, or both, it is advisable to switch toward a different opioids [45]. Transdermal opioids may be effective alternatives in patients unable to swallow [45].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree