Robert E. Coleman and Ingunn Holen • Bone metastasis is a major cause of (often prolonged) morbidity in persons with cancer. • Bone metastasis is especially prevalent in persons with breast and prostatic cancer. • Tumor cells are attracted to particular niches in bone and may remain dormant for prolonged periods, as controlled by microenvironmental cues. • Osteolytic damage is mediated largely by stimulation of osteoclasts via tumor-derived cytokines; intermediary cells, including immune cells and osteoblasts, are involved. • Differential diagnosis includes osteoporosis, degenerative disease, and Paget disease. • The isotope bone scan is a sensitive test to detect the presence of skeletal pathology but gives little information about its nature. • Structural information on skeletal damage from metastatic bone disease is best obtained by skeletal radiography supplemented with computerized tomography and magnetic resonance imaging. • Positron emission tomography provides functional information that may aid in diagnosis. • An assessment of the patient’s symptoms and activity status is essential. • Skeletal radiography assesses response to treatment, but the information is delayed and the method is insensitive. • Early indications of response of bone metastases to treatment can be obtained by monitoring biochemical markers of bone metabolism. • Isotopic bone scanning is not useful in monitoring response to treatment. • Antitumor treatments such as external beam radiotherapy, endocrine therapy, cytotoxic chemotherapy, targeted biological agents, and radioisotope therapy have a role in the multidisciplinary palliative treatment of metastatic bone disease. • The bisphosphonates and the receptor activator of nuclear factor κ ligand inhibitor denosumab are inhibitors of osteoclast activity and have become important agents for the treatment of metastatic bone disease because they relieve symptoms, allow bone healing, and delay complications. • Complications include pain, impaired mobility, pathological fracture, spinal cord compression, cranial nerve palsies, nerve root lesions, hypercalcemia, and suppression of bone marrow function. • The treatment of choice for hypercalcemia is bisphosphonates in combination with intravenous rehydration. • Orthopedic surgery has an important role in the treatment and prophylaxis of pathological fractures and spinal decompression and stabilization to relieve spinal cord compression or instability. Primary bone cancer occurs predominantly among children and adolescents and is rare. By contrast, secondary bone cancer—particularly from carcinomas of the breast, lung, prostate, kidney, and thyroid—is common. The incidence of bone metastases from different primary sites recorded in postmortem studies is summarized in Table 51-1. Although the variability in these metastatic patterns is probably related to molecular and cellular biologic characteristics of both the tumor cells and those of the tissues to which they metastasize, other factors, such as vascular pathways and blood flow, are also important.1 Table 51-1 Incidence of Skeletal Metastases from Autopsy Studies Modified from Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12(Suppl.):6243–9. Breast cancer is the most common malignancy in most parts of the world, accounting for some 10% to 15% of all cancers, and it is the tumor most often associated with metastatic bone disease. Because of the potentially long clinical course of this disease, even after metastases have developed, morbidity from bone deposits presents a major problem for health care systems. Approximately 70% of patients dying from breast cancer will have radiologic evidence of skeletal metastases before death, and bone is the first metastatic site in more than 40% of persons with distant relapse. Median survival after a first relapse in bone is around 2 to 3 years and is significantly longer than that seen after a first relapse in visceral sites such as the liver.1 Although the management of metastatic visceral disease has improved in recent years, the prognosis after visceral relapse remains relatively poor, whereas bone metastases can be a protracted problem for many years. Favorable factors for a longer survival after first relapse in bone are low histologic grade, positive estrogen receptor (ER) status, long postoperative disease-free interval, and lack of development of extraosseous disease.2 The skeleton is by far the most common site of metastatic disease in persons with prostatic cancer. Unlike breast cancer, in which the lesions frequently show a mix of osteoblastic and osteolytic appearances radiologically, osteosclerotic disease predominates in prostate cancer. Nevertheless, computed tomography (CT) often identifies lytic areas within these ostensibly sclerotic lesions. Histologic and biochemical studies also have demonstrated increased bone resorption in metastatic prostate cancer.3 As in breast cancer, metastatic bone disease in persons with prostate cancer can follow a relatively long course over several years. Several studies have attempted to correlate the extent of skeletal metastatic involvement with survival in patients with advanced prostate cancer. A system based on the number of lesions identified by bone scintigraphy was predictive for survival,4 and a bone scan index to more accurately estimate the extent of skeletal involvement by tumor has been developed. When this index was used in 191 patients with androgen-independent prostate cancer, patients with low, intermediate, or extensive skeletal involvement had median survivals of 18.3, 15.8, and 8.1 months, respectively.5 Interest in personalized medicine is increasing, whereby biologic factors are used to predict the probability or distribution of recurrence. The association between ER expression and bone metastasis from breast cancer has been appreciated for >20 years.1 However, other markers, especially the noncollagenous bone proteins, have more recently been suggested as predictors of bone recurrence. These markers have included bone sialoprotein6,7 and C-terminal propeptide of type I collagen.8 Parathyroid hormone–related peptide (PTHrP) is expressed by a very high proportion of breast cancers in bone, but paradoxically, expression of PTHrP in the primary tumor is associated with a better prognosis and a lower incidence of metastasis.9 Gene expression profiling has identified genetic signatures that are strongly associated with prognosis, possibly outperforming conventional panels of clinical and pathologic criteria. However, the risk of metastasis per se does not necessarily predict for metastasis to specific sites. Several gene signatures have been described that appear predictive of metastasis to bone as opposed to dissemination to other sites.10 However, much more work is required to confirm the usefulness of this use of genetic profiling in clinical management. Our view of the role of the primary tumor in metastatic growth fundamentally changed with the introduction of the concept of the “premetastatic niche.”11 In model systems, factors released by the primary tumor were found to direct migration of hematopoietic progenitor cells from the bone marrow to cluster in particular sites for future metastatic growth, preparing the “soil” to create a suitable environment for subsequent colonization by tumor cells. This model supports the notion that initiation of metastatic spread is an early event, probably occurring before the primary tumor becomes clinically detectable, rather than being associated with high tumor burden. The current view is that after leaving the primary site, tumor cells are attracted to particular “metastatic niches” in the hematopoietic bone marrow and that tumor cells may subsequently spread from this bone niche to other organs.12 The precise characteristics of the metastatic niche remain to be established, but prostate cancer models provide convincing evidence to support the notion that tumor cells occupy the hematopoietic stem cell niche.13 Once established in the niche, tumor cells may remain dormant for many years because of the control of the surrounding bone microenvironment. Bone is a highly specialized connective tissue comprising an unmineralized (osteoid) matrix composed predominantly of type I collagen and a mineralized component of hydroxyapatite crystals, which encloses the marrow space that contains a variety of marrow-residing cells (including osteoblasts, osteoclasts, bone marrow stromal cells, immune cells, mesenchymal stem cells, adipocytes, fibroblasts, and endothelial cells), platelets, fat, and interstitial fluid. During childhood and adolescence, bone is constantly shaped and remodeled, with peak bone mass being reached in early adulthood. Bone turnover continues throughout life, with up to 20% of the skeleton undergoing remodeling at any time to replace damaged bone and maintain skeletal integrity. The total duration of a remodeling cycle in young adults is estimated to be around 200 days. This process is essential for providing bone strength and is responsive to mechanical stress.14 Bone resorption is mediated by the osteoclast, a multinucleated giant cell derived from granulocyte-macrophage precursors, whereas bone formation is carried out by osteoblasts, which are derived from mesenchymal fibroblast-like cells. In a healthy person, bone resorption and bone formation are coupled and perfectly balanced in location, time, and amount. Bone remodeling is regulated by complex interactions between hormones, paracrine growth factors, and cytokines and involves interactions between osteoclasts and osteoblasts, as well as other cell types present in the bone microenvironment (Fig. 51-1). Recent studies in model systems have shown that direct contact with breast cancer cells cause substantial changes to the surrounding bone microenvironment at early stages, prior to the formation of bone lesions.15 The presence of tumor cells ultimately results in the development of cancer-induced bone disease that disrupts the fine-tuned balance between osteoblast and osteoclast activity, either causing excess bone resorption (as associated with lytic bone lesions), or increased levels of bone formation (as in osteosclerotic bone lesions) (Fig. 51-2). In many cases mixed lesions are present, which have both lytic and sclerotic features. In the late stages of cancer, tumor masses may also damage the skeleton by compression of vasculature and consequent ischemia. Malignant cells secrete factors that stimulate osteoclastic activity both directly and indirectly, and the importance of interactions between malignant cells and the bone microenvironment in the development of bone metastases has been the focus of intensive research, as summarized in a recent review.16 Factors produced by tumor cells that cause increased bone resorption include prostaglandin E and a variety of cytokines and growth factors, such as transforming growth factor (TGF) α and β, epidermal growth factor, vascular endothelial growth factor, tumor necrosis factor (TNF), and interleukin (IL) 1, 6, 8, and 11 (Fig. 51-3). Several proteolytic enzymes are proposed to be involved in the early phases of formation of bone metastases, including matrix metalloproteinases (MMPs; e.g., MMP-2 and MMP-9) and cathepsin K.17 Normal bone trabeculae are lined by a thin layer of uncalcified matrix, which protects the calcified bone from osteoclastic activity, and the action of proteolytic enzymes might be a prerequisite for osteoclastic bone resorption. In addition, MMPs are involved in formation of bone metastases both through their ability to degrade basement membrane and facilitate tumor cell dissemination but also by causing release of growth factors and cytokines bound to the bone matrix, thereby supporting further tumor cell proliferation.18 Malignant cells might also increase bone resorption by stimulating tumor-associated immune cells to release osteoclast-activating factors. It has been shown that human melanoma cells produce a factor that stimulates macrophages to release TNF and IL-1 in vitro. Furthermore, purification of a cytokine-releasing factor from medium conditioned by melanoma cells has identified it as granulocyte-macrophage colony-stimulating factor, which activates osteoclastic bone resorption.19 In addition to the local paracrine factors just described, osteoclastic activity can also be stimulated in malignant disease by systemic factors, particularly PTHrP.20 This peptide is immunologically distinct from parathyroid hormone, but the two hormones have significant homology at the amino terminus of the molecule, which is necessary for osteoclast stimulation. Ectopic production of this hormone, particularly in persons with lung cancer, is a cause of osteoclastic bone resorption and hypercalcemia even in the absence of bone metastases. The importance of PTHrP in the pathogenesis of osteolysis induced by metastatic breast cancer has been elegantly elucidated.20 In bone metastases, the secreted PTHrP acts in a paracrine manner to stimulate osteoclastic bone resorption, which not only leads to skeletal damage but also appears to confer a selective growth advantage on the tumor cells. This advantage is conveyed by the release of factors for tumor growth from bone during the osteolytic process. Hence a vicious circle is established in which the growth of metastatic cells is potentiated, leading to enhanced osteolysis and secretion of further growth factors to stimulate tumor cell proliferation. This paracrine activity occurs even in the absence of hypercalcemia or increased circulating PTHrP levels. PTHrP stimulates osteoclastic resorption by increasing osteoblast and stromal cell production of receptor activator of nuclear factor–κB (RANK) ligand (RANKL). RANKL binds to its receptor, RANK, on osteoclast lineage cells, resulting in differentiation to mature osteoclasts and stimulation of osteoclast activity. In the normal bone environment, osteoblast secretion of osteoprotegerin (OPG) neutralizes RANKL, terminating its stimulatory effects on osteoclasts.21 The MCF-7 estrogen-dependent breast cancer cell line has been found to decrease osteoblastic OPG messenger RNA levels, enhancing osteoclast formation. This imbalance is compounded by release of TGF-β and insulin-like growth factor (IGF1) from resorbing bone, which further promotes tumor production of PTHrP, perpetuating the cycle of osteolytic bone destruction. The OPG-RANK-RANKL triad of molecules are central not only to normal bone physiology but have a key role in the development of bone metastasis, with several different tumor cell types producing one or more of these factors.22 Although osteolytic disease is usually most evident at sites of bone metastases, osteosclerosis sometimes can predominate, particularly in prostate cancer. Prostate cancer, in contrast to breast cancer, tends to cause osteoblastic lesions in bone, leading to dense, sclerotic-looking metastases on plain radiographs, and osteoblast growth factors such as TGF-β and platelet-derived growth factor have been purified from prostatic tumor cells. One of the factors that might be involved in prostatic bone lesions is the growth factor endothelin-1 (ET-1), which is produced by prostate cancer cells.23 Circulating levels have been found to be increased in patients with osteoblastic bone metastases from androgen-refractory prostate cancer, compared with patients whose cancer is confined to the prostate and normal control subjects. ET-1 has been found to stimulate osteoblast activity in animal models and to inhibit osteoclast activity, whereas antagonists of endothelin have been found to inhibit bone formation in vivo.24 Furthermore, a recent study found that ET-1 production by prostate cancer cells is reduced by androgens but is stimulated in androgen-insensitive prostate cancer cells by factors such as TGF-β.25 This finding is clinically relevant, because metastatic prostate cancer typically develops androgen resistance. It is possible that PTHrP is also involved in the pathogenesis of prostate cancer bone metastases, because co-expression of PTHrP and its receptor has been found in both the primary tumor and in bone metastases of patients with prostate cancer.26 Evidence from morphometric studies and measurement of urinary markers of osteolytic bone resorption has led to the hypothesis that osteoclastic bone resorption initially is important in prostate cancer and is followed by intense osteoblastic activity.27 This scenario might not always be the case, however. When the prostate cancer cell line PC-3 is implanted into the tibia of severe combined immunodeficiency (SCID) mice, osteolytic lesions develop, possibly through secretion of RANKL.28 However, when another prostate cancer cell line (LAPC-9) was used, osteoblastic lesions developed even when no osteoclasts were present. Therefore these authors concluded that osteoclastic activity might not be a prerequisite for the formation of osteoblastic lesions. Interest is increasing in the role of the osteoblasts in bone metastases, and it has recently been established that the Wnt signaling system plays a key role in bone development and turnover.29 Subsequent studies have found that several molecules in this system are implicated in the development of bone metastases.30 In prostate cancer, tumor-derived Wnt induces osteoblastic activity in bone metastases, which in the early stages of disease may be counteracted by the presence of the Wnt agonist dickopf-1 (DKK1), thereby favoring lytic lesions. In the later stages of progression of prostate cancer bone metastases, the balance between Wnt and its inhibitors appears to be shifted toward Wnt, favoring osteoblastic lesions. In myeloma bone disease, bone marrow stromal cells are important for the pathogenesis of multiple myeloma. IL-6 appears to be an important growth and survival factor for myeloma cells and for conferring resistance to treatment with dexamethasone, a commonly used treatment for multiple myeloma. MMPs, which are known to be important in normal and malignant remodeling, also contribute to the pathogenesis of myeloma. Bone marrow stromal cells secrete interstitial collagenases (MMP-1) and gelatinase A (MMP-2). MMP-1 initiates bone resorption, degrading type I collagen, which becomes a substrate for MMP-2. Malignant plasma cells have been found to upregulate MMP-1 and activate MMP-2.31 Dysregulation of the Wnt signaling system has also been implicated in myeloma bone disease. The production of DKK1 (an inhibitor of osteoblast differentiation) by myeloma cells is reported to be associated with the presence of lytic bone lesions,32 whereas inhibition of DKK1 prevents the development of lytic bone disease in models of multiple myeloma.33 In all cases, imaging tests are necessary and must be interpreted in conjunction with the clinical picture and—when appropriate—serum tumor markers. Occasionally, performing a bone biopsy under radiologic control or through an open operation is necessary. The typical clinical, radiologic, bone scan, and biochemical abnormalities of the more common skeletal pathologies are summarized in Table 51-2. Table 51-2 Clinical, Radiologic, and Biochemical Features of Common Skeletal Disorders That May Mimic Bone Metastases Lytic metastases are the most common type arising from breast, lung, thyroid, renal, melanoma, and gastrointestinal malignancies (Fig. 51-4). Thinning of trabeculae occurs, and the margins are usually ill defined, representing regions of partially destroyed trabeculae between the central destruction and the radiologically normal bone. The width of the margin reflects the aggressiveness of the lesion, with a narrow zone of transition in the less aggressive lesions. If the metastasis is in the medulla, endosteal scalloping could be present, whereas cortical lesions produce subperiosteal scalloping or a focal cortical defect. Sclerotic metastases are usually from prostate cancer but also arise from breast, lung, and carcinoid tumors (Fig. 51-5). Excessive new bone formation gives rise to thickened, coarse trabeculae, which usually appear on radiograms as nodular, rounded, fairly well-circumscribed sclerotic areas. Sometimes less well-defined, mottled, irregular areas of increased bone density appear, which can coalesce to produce a diffuse sclerotic appearance to the skeleton. A bone scan is generally performed by acquiring multiple images of the skeleton 3 to 4 hours after the intravenous injection of technetium-99m–labeled bisphosphonate. If a lesion is identified, further investigation is necessary, particularly when it is solitary. A suggested protocol for investigation is shown in the accompanying algorithm (Fig. 51-6). Appropriate plain radiographs of a focal lesion should be obtained in the first instance. If findings of these radiographs are normal and yet clinically a metastasis is likely, CT or magnetic resonance imaging (MRI) of the area could be diagnostic. Although bone scan appearances are nonspecific, recognizable patterns of bone scan abnormalities might suggest a specific diagnosis. Metastases are usually multiple, irregularly distributed foci of increased tracer uptake that do not correspond to any single anatomic structure (Fig. 51-7). Generally they affect the axial skeleton, but metastatic disease can involve the appendicular skeleton, and approximately 7% of patients have involvement of the distal skeleton, with the proportion increasing in certain tumor types such as renal cell carcinoma.34 Because detection of a lesion depends on the presence of a focal increase in osteoblast activity, a false-negative scan will occur when pure lytic disease is present. A false-negative scan is typical of multiple myeloma, which is best investigated radiographically, but a false-negative scan also can occur in other tumors when rapidly growing lytic lesions are present. In extreme cases where significant bony destruction has occurred, a photon-deficient area (cold spot) can develop (Fig. 51-8). Sclerotic metastases, on the other hand, are generally clearly visualized on the bone scan, with the only exception being very slow-growing metastases, in which the alteration in metabolic activity is so subtle that it might not be distinguishable from normal background activity. When extensive skeletal metastases are present, the focal lesions can coalesce to produce diffusely increased uptake—the so-called “super scan” of malignancy (Fig. 51-9). This scenario occurs most often in prostate cancer but also is seen in other tumors, such as breast cancer. Increases in the contrast between bone and background soft tissue and faint or absent renal images are the typical appearances. A CT scan produces images with excellent soft tissue and contrast resolution. Bony destruction and sclerotic deposits are well visualized (Fig. 51-10), and any soft tissue extension of bone metastases is demonstrated clearly. CT can help with the diagnosis of spinal metastases and is particularly useful to localize lesions for biopsy.35 MRI permits imaging of the entire spine in the sagittal plane (Fig. 51-11). With a T1-weighted scan acquisition, the solid constituents of cortical bone give no signal on MRI and appear black, whereas the high water content of fat and bone marrow results in strong signals, making these tissues appear white. This signal is variable, depending on the pulse sequence used. Detection of bone metastases by MRI depends on differences in MR signal intensity between tumor tissue and normal bone marrow. Metastatic tumor is therefore visualized directly, in contrast to the indirect changes observed by x-ray or radionuclide bone scanning. Like CT, MRI has proved useful for evaluating patients with positive bone scans and normal radiograms and for elucidating the cause of a vertebral compression fracture. MRI is excellent for demonstrating bone marrow infiltration and is more sensitive than a bone scan for the early detection of spinal metastases.36 Scanning with fluorine 18–labeled fluorodeoxyglucose (18F-FDG)-positron emission tomography (PET) provides the opportunity to visualize function. However, its role in the routine identification of bone metastases is far from clear. FDG has the advantage of demonstrating all metastatic sites, and in the skeleton it is assumed that its uptake occurs directly into tumor cells. For bone metastases from breast and lung cancer, FDG-PET has similar sensitivity, though poorer specificity, as the isotope bone scan. FDG-PET is less sensitive than a bone scan for prostate cancer, whereas for multiple myeloma (presumably because FDG is identifying marrow-based disease at an early stage), FDG-PET is clearly better than a bone scan.36 The effects of tumor cells on bone cell function can influence serum and urinary levels of biochemical markers of bone metabolism. In recent years, the number of available markers and the clinical relevance has increased rapidly; their value in the diagnosis of bone metastases has been studied in several tumor types. Table 51-3 lists some of the currently available bone resorption and formation markers. Their potential roles in malignancy have been extensively reviewed.39–39 Table 51-3 Biochemical Markers of Bone Resorption and Formation Because commonly used radiographic methods do not detect bone metastases in the very early stages of development, the question arises whether serial measurements of bone markers might identify the impending development of bone metastases. Thus far, data relating to this question are inconclusive. Elevated levels of bone markers, notably bone sialoprotein,6,7 N-terminal propeptide of procollagen type 1,40 and C-terminal telopeptide of type 1 collagen,8 have been shown to have prognostic value; patients with levels above a defined threshold had a significantly greater probability of bone recurrence. However, in an individual patient, bone marker levels are unlikely to be particularly useful in making a diagnosis of metastatic bone disease before there is imaging evidence or helping to establish the nature of a solitary imaging abnormality. Once bone metastases have developed, bone markers can provide useful prognostic information. Clinical evidence of correlations between bone marker levels and patient outcomes have been reported from retrospective analyses of several large trials in patients with bone metastases.3,41 Elevated bone marker levels during the course of the study correlated with negative clinical outcomes from a range of solid tumors or myeloma. Specifically, patients with elevated urinary N-telopeptide of type I collagen (NTX) levels either at baseline or at their most recent assessment had an approximate twofold increase in their risk of disease progression, an approximate two- to threefold increase in their relative risk of skeletal-related events (SREs) compared with patients with low NTX levels, and a two- to fivefold increased risk of death. In contrast, other than in prostate cancer, bone-specific alkaline phosphatase levels were not a consistently strong prognostic indicator.3 NTX levels have also been shown to provide valuable prognostic information in a larger analysis of patients receiving bisphosphonate treatment for bone metastases from a wide range of solid tumors and multiple myeloma.41 Among patients with solid tumors, elevated NTX levels were associated with a twofold increased risk of disease progression (P < .001). Furthermore, elevated NTX levels were associated with a four- to sixfold increased risk of dying upon study in all patients with solid tumors (P < .001 for all). A variety of treatments including radiotherapy, endocrine treatment, chemotherapy, and bone-targeted agents are used for the treatment of metastatic bone disease, and evaluation of their effects is important for both routine clinical practice and research. The current imaging methods used to assess response to these treatments are qualitative and routinely include plain radiographs, radionuclide bone scans, and, in particular situations, CT scans. Assessing the response of bone metastases to therapy is notoriously difficult; the events in the healing process are slow to evolve and are quite subtle, with sclerosis of lytic lesions only beginning to appear 3 to 6 months after the start of therapy and taking more than a year to mature.42 Bone is the only site of metastatic disease that has separate criteria for evaluation of response to treatment, based on bone repair and destruction rather than on changes in tumor volume. A complete review of the bone radiographs since the start of treatment is necessary to evaluate response, which is a slow and imprecise process. Radiologic assessment of response is based on radiographic evidence of bone healing.42 It is generally accepted that sclerosis of lytic metastases with no radiologic evidence of new lesions constitutes tumor regression (a partial response). Confounding factors include the appearance of sclerosis in an area that was previously normal on the radiograph. This finding could represent progression of a new metastasis but could also indicate a response, reflecting a radiographic example of the healing flare phenomenon within a lesion that was present at the start but was not destructive enough to be radiographically visible. Interpretation is further complicated by variations in film exposure and the effects of overlying bowel gas. The evaluation of response in osteosclerotic lesions is even more difficult; most patients with sclerotic metastases are eventually classified as either having “no change” in response to therapy or as being unassessable. In these cases, decisions about the efficacy of treatment must be based on symptomatic response, changes in tumor markers, or (when present) change in extraskeletal disease. Although we recognize that the changes seen on serial radiograms remain the “gold standard” for evaluating response to therapy, new methods of assessing response are needed, both to improve patient management and to evaluate specific treatments. Several alternatives or adjuncts to assessment based on plain radiograms have been suggested.38,42,43 None is ideal, each having advantages and disadvantages as outlined in the following sections. The relief of symptoms is the principal aim of palliative therapy and rationally should be the most important marker of response to treatment. The use of pain as a marker of response in clinical trials has not found universal acceptance, however, and there is still no single internationally accepted pain questionnaire in the field of oncology, although the Brief Pain Inventory is frequently used in clinical trials. Subjective response to treatment for bone disease requires information on pain intensity, analgesic consumption, and mobility.42 Quality of life (QOL) assessment is now an important aspect of clinical trials methodology and, notwithstanding all the difficulties of analysis and interpretation, well-validated generic tools such as the Functional Living Index in Cancer patients and the European Organization for Research and Treatment of Cancer (EORTC) QOL-C30 questionnaire can provide useful information on subjective response to treatment in the routine clinical setting. A specific QOL tool for patients with bone metastases has recently been validated (EORTC-BM22)44 and could be considered for assessment of patient outcomes in routine clinical practice and in clinical trials. Although the plain radiograph remains the assessment tool used to judge response in many clinical trials, it is clearly an inadequate technique. The lack of precision for radiographic assessment of response is exemplified by the observation that, in terms of survival, patients with radiographic evidence of sclerosis (partial response) and patients with no change in radiographic appearance for at least 3 months have a similar outcome.42 The use of bone scanning for assessment of response to therapy has always been contentious and is certainly unreliable when lytic metastases predominate. A reduction in the intensity and number of lesions (hot spots) on the bone scan was previously considered to represent response, and progressive disease was assumed if an increase in intensity or number of hot spots was seen. This interpretation is too simplistic, however. After successful therapy for metastatic disease, the healing processes of new bone formation cause an initial increase in tracer uptake (akin to callus formation), and scans performed during this phase are likely to show increased intensity and an increased number of hot spots. After treatment for 6 months, the bone scan appearances might improve as the increased production of immature new bone ceases and isotope uptake gradually falls. This “deterioration” followed by subsequent “improvement” in the bone scan appearances after successful therapy has been termed the “flare response” and is now a well-recognized phenomenon in both breast and prostate cancers (Fig. 51-12). Conversely, a reduction in isotope uptake can occasionally be seen in rapidly progressive disease, when overwhelming bone destruction allows little chance for new bone formation, sometimes culminating in a photon-deficient (cold spot) lesion on the bone scan. The role of PET scanning in assessment of response has become clearer in recent years.45 The regular, well-defined tumor margins that are necessary for reproducible anatomic measurements are of lesser importance in functional imaging. Functional imaging criteria, such as the recently developed Positron Emission Tomography Response Criteria in Solid Tumors, have allowed response to be measured in the absence of anatomic change through assessment of metabolic activity but have not yet been accepted for routine use.45 In breast cancer, unlike in germ cell malignancies, no highly specific tumor marker is available for either diagnosis or monitoring of disease. However, some breast tumors do produce tumor antigens that can be detected by radioimmunoassay. The most widely studied are carcinoembryonic antigen, which is elevated in 50% to 80% of patients with metastatic breast cancer, and carcinoma antigen (CA) 15-3, which is elevated in 60% to 90% of persons with advanced disease. Dynamic changes must be interpreted with caution. Although some patients show the expected increase in markers with progression and decrease in markers with regression, others who respond show an initial surge in tumor marker followed later by the expected decline. A combination of carcinoembryonic antigen, CA 15, and erythrocyte sedimentation rate may provide an indication of response. In a study in patients with metastatic breast cancer, a significant correlation with clinical assessment of response was found using Union Internationale Contre Cancer criteria at 2, 4, and 6 months after systemic endocrine treatment. A multicenter evaluation of the same index confirmed that changes in the markers were in line with and often predated therapeutic outcome criteria for both remission and progression.46 Prostate-specific antigen (PSA) is a marker of prostatic pathology and can be elevated in any prostate disease, although the highest tissue production occurs in prostate cancer. PSA has proved useful in the early diagnosis, staging, and follow-up of patients.47 The level of PSA is dependent on the volume of cancer, the volume of benign prostatic hypertrophy in the prostate, and the differentiation of the tumor, with less production of PSA from poorly differentiated tumors. PSA levels usually decline after androgen deprivation therapy (ADT) is initiated and generally provide a reliable guide to response; elevations of PSA usually antedate other clinical evidence of progression by at least 12 months.48 The PSA doubling time is a useful predictor of overt metastases in castrate-resistant prostate cancer (CRPC)48 and has been used to select suitable patients to enable evaluation of new treatments developed to delay or prevent metastasis. The stimulation of osteoclast function that results in osteolysis and disruption of the normal coupling between osteoblast and osteoclast function leads to changes in a variety of biochemical parameters. When treatment is prescribed for a patient with bone metastases, the effects of that treatment on the tumor cell population will influence bone cell activity. These changes can be appreciated within the first few weeks of starting effective therapy; for this reason, biochemical markers that reflect the rates of bone formation and resorption, respectively, can provide an early assessment of response to treatment.38,39 More recently, attention has turned to the possible use of collagen cross-link measurements for the assessment of response in bone. Several pilot studies have shown that bone resorption markers will usually fall if disease is controlled, whereas bone formation markers show an initial rise in the first 1 to 3 months of successful treatment because of the flare response followed by a fall as the response is consolidated.39,42 Although it is now well accepted that bone-targeted systemic therapy—particularly bisphosphonates—can reduce morbidity of skeletal metastases of breast cancer substantially, the optimization and timing of these therapies remain to be established. Bone markers potentially offer a powerful and relatively simple tool to assist the clinician in developing the most appropriate treatment strategies. Moreover, there is a prospect of using bone markers to tailor treatment to the individual patient. However, this prospect remains a research question, and use of bone markers is not recommended by current guidelines.49,50 Evidence indicates that the individual pretreatment values of a bone marker correlate with response to treatment. In patients with metastatic bone pain, normalization of bone resorption markers correlated with response to treatment.51 Clinical benefit, as indicated by an improvement in a pain score, was seen only among the patients achieving a normal bone resorption rate after administration of pamidronate. No response was seen in the patients with persistently elevated levels, which suggested that the aim of bisphosphonate therapy should be to produce a fall in marker levels, preferably into the normal range. A subsequent study has shown that this principle may be extended to the use of bone markers to distinguish between the benefits of different bisphosphonates.52 The ability of bisphosphonate therapy to reduce the frequency of skeletal complications also seems to be correlated with a reduction in bone resorption markers. Retrospective analyses of large randomized trials with zoledronic acid have shown that among patients with solid tumors, elevated NTX levels despite treatment were associated with a significant two- to threefold increased risk of both skeletal complications and death (P < .001).53 In general, the treatment of bone metastases is aimed at palliating symptoms, with cure only rarely a realistic aim (e.g., in lymphoma); treatment varies depending on the underlying disease. External beam radiotherapy, endocrine treatments, chemotherapy, and radioisotopes are all important. In addition, orthopedic intervention may be necessary for the structural complications of bone destruction, and some patients with bone metastases experience hypercalcemia, which requires specific treatment (see Chapter 37). Optimal management requires a multidisciplinary team that includes not only medical and radiation oncologists, orthopedic surgeons, general physicians, radiologists, and nuclear medicine physicians but also palliative medicine specialists and the symptom control team. A schema for the management of bone metastases is shown in the accompanying algorithm (Fig. 51-13). Treatment decisions depend on whether the bone disease is localized or widespread, the presence or absence of extraskeletal metastases, and the nature of the underlying malignancy. Radiotherapy is frequently relevant throughout the clinical course of the disease. Resistance to systemic treatments can be expected to develop, necessitating periodic changes of therapy in an effort to regain control of the disease.
Bone Metastases
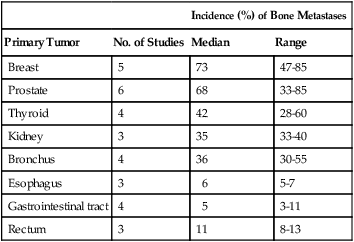
Incidence
Primary Tumors Leading to Bone Metastases
Incidence (%) of Bone Metastases
Primary Tumor
No. of Studies
Median
Range
Breast
5
73
47-85
Prostate
6
68
33-85
Thyroid
4
42
28-60
Kidney
3
35
33-40
Bronchus
4
36
30-55
Esophagus
3
6
5-7
Gastrointestinal tract
4
5
3-11
Rectum
3
11
8-13
Causes
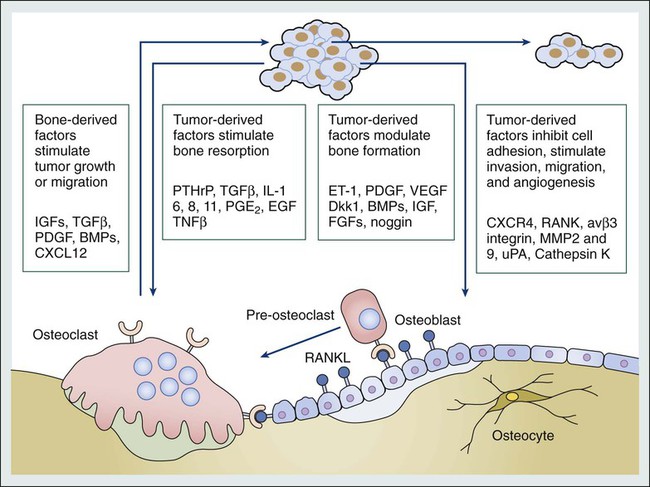
Mechanisms of Metastases
Pathogenesis
Bone Remodeling
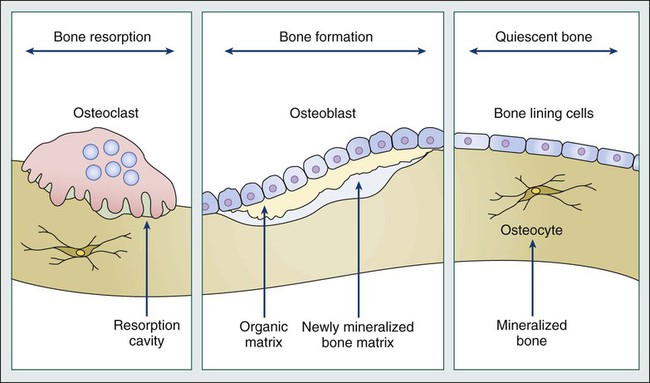
Tumor Cell-Bone Cell Interactions
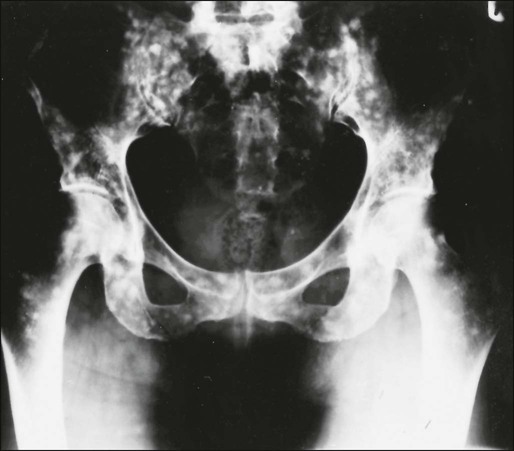
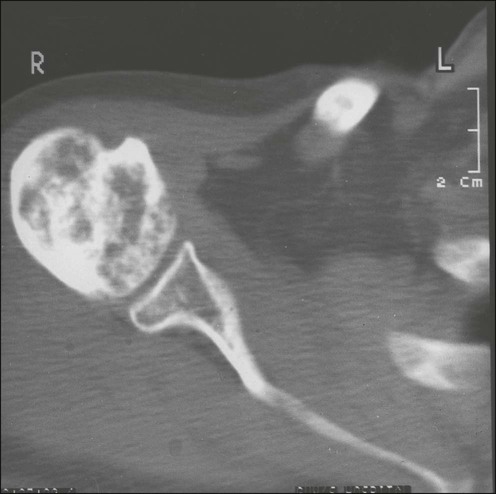
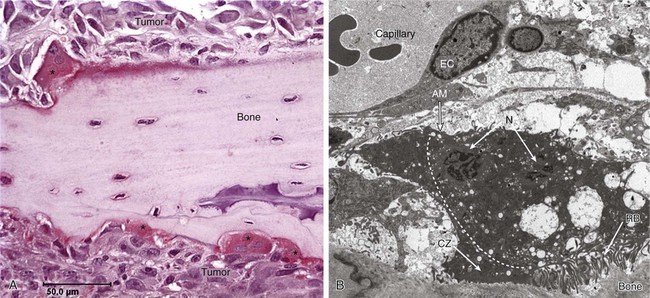
Sclerotic Bone Metastases
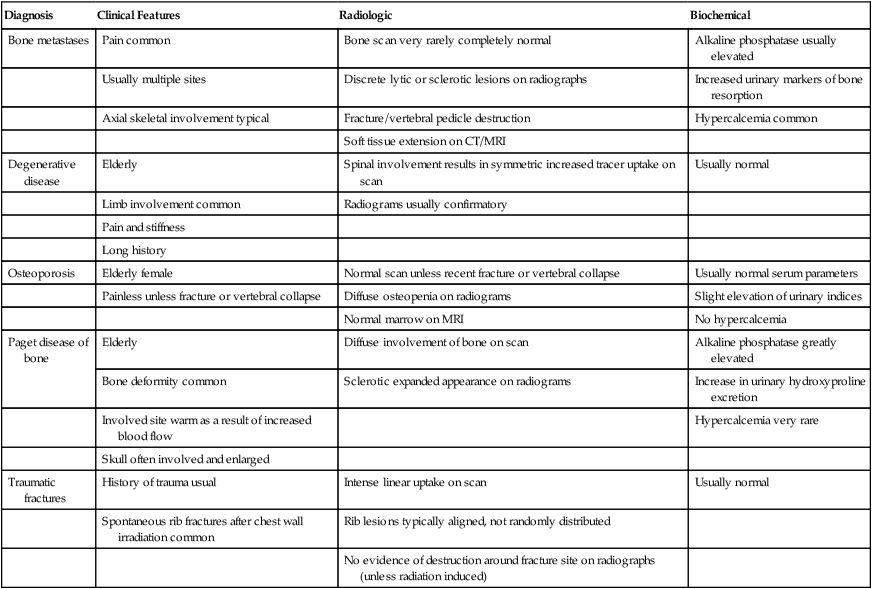
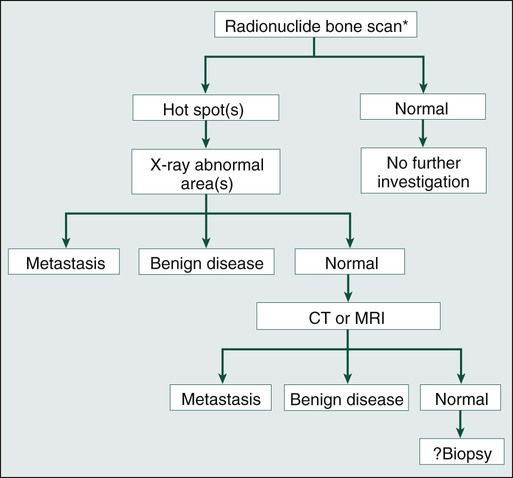
Diagnosis
Differential Diagnosis
Diagnosis
Clinical Features
Radiologic
Biochemical
Bone metastases
Pain common
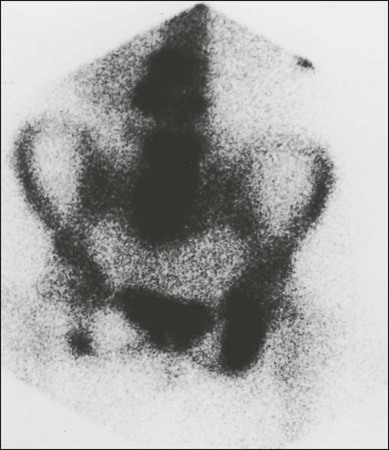
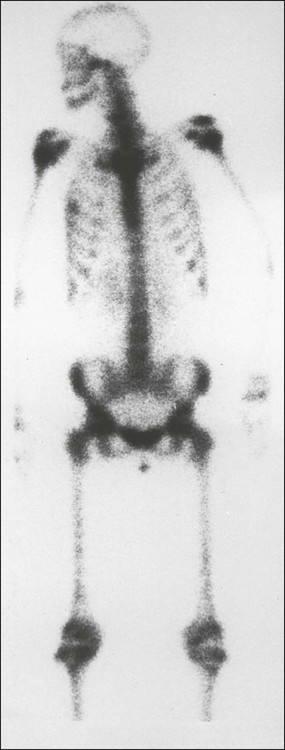
Bone scan very rarely completely normal
Alkaline phosphatase usually elevated
Usually multiple sites
Discrete lytic or sclerotic lesions on radiographs
Increased urinary markers of bone resorption
Axial skeletal involvement typical
Fracture/vertebral pedicle destruction
Hypercalcemia common
Soft tissue extension on CT/MRI
Degenerative disease
Elderly
Spinal involvement results in symmetric increased tracer uptake on scan
Usually normal
Limb involvement common
Radiograms usually confirmatory
Pain and stiffness
Long history
Osteoporosis
Elderly female
Normal scan unless recent fracture or vertebral collapse
Usually normal serum parameters
Painless unless fracture or vertebral collapse
Diffuse osteopenia on radiograms
Slight elevation of urinary indices
Normal marrow on MRI
No hypercalcemia
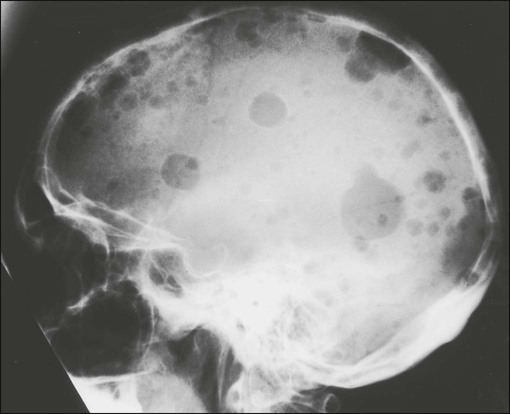
Paget disease of bone
Elderly
Diffuse involvement of bone on scan
Alkaline phosphatase greatly elevated
Bone deformity common
Sclerotic expanded appearance on radiograms
Increase in urinary hydroxyproline excretion
Involved site warm as a result of increased blood flow
Hypercalcemia very rare
Skull often involved and enlarged
Traumatic fractures
History of trauma usual
Intense linear uptake on scan
Usually normal
Spontaneous rib fractures after chest wall irradiation common
Rib lesions typically aligned, not randomly distributed
No evidence of destruction around fracture site on radiographs (unless radiation induced)
Diagnostic Methods
Skeletal Radiography
Radionuclide Bone Scan
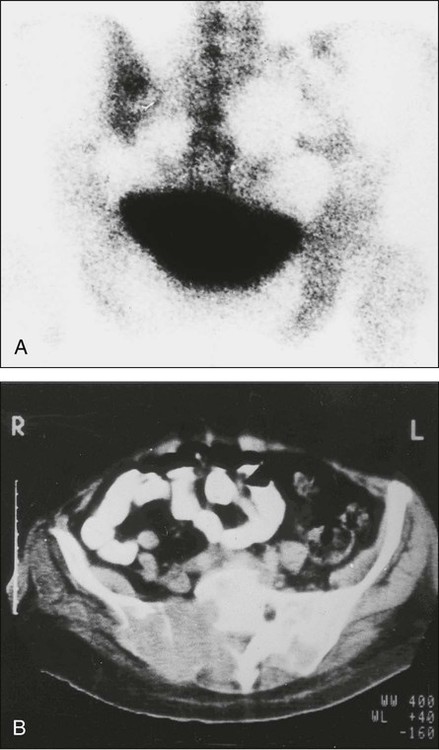
Computed Tomography
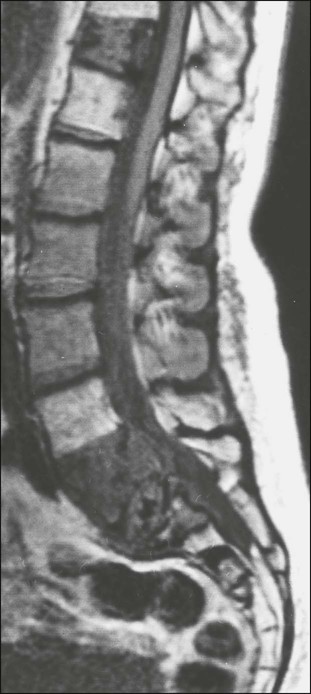
Magnetic Resonance Imaging
Positron Emission Tomography
Biochemical Markers of Bone Metabolism
Resorption
Formation
URINE
SERUM
Calcium
Alkaline phosphatase
Hydroxyproline
Osteocalcin
PYD
PICP
DPD
PINP
NTX
Crosslaps
Free DPD
Free PYD
Galactosyl hydroxylysine
SERUM
Calcium
ICTP
Galactosyl hydroxylysine
Bone Markers as Predictive and Prognostic Indicators
Evaluation of the Patient
Assessment of Symptoms and Activity Status
Imaging of Bone Metastases
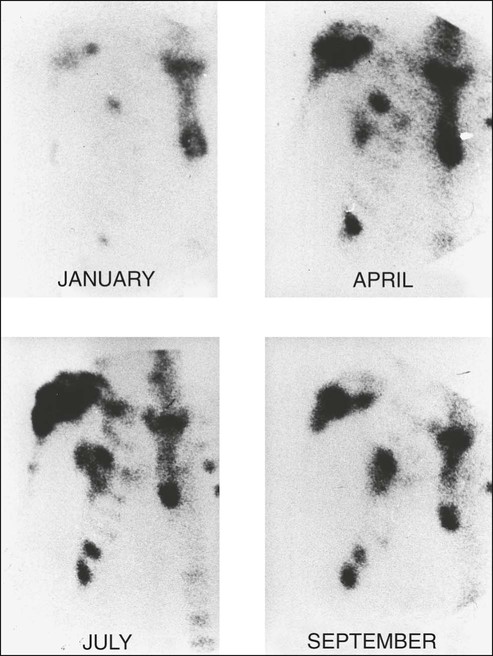
Tumor Markers
Biochemical Assessment of Response
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine