This article addresses current transplant options for patients with acquired severe aplastic anemia (SAA). This discussion includes ongoing progress in the use of SAA in the setting of unrelated donor transplants, which now provide outcomes similar, though still not identical, to HLA-identical sibling transplants. Recent data on stem cell source, conditioning regimens, and graft-versus-host disease prophylaxis are outlined. Other donor types such as cord blood and haploidentical mismatched family donors are also discussed.
Key points
- •
Stem cell transplantation for acquired aplastic anemia has made significant progress over the past decade, and this is true especially for alternative donor transplants, including unrelated, cord blood, and haploidentical grafts.
- •
Significant predictors of survival remain the age of the patient and the interval between diagnosis and transplants: young patients with a short interval have the best survival.
- •
Early referral to experienced centers is thus mandatory for this rare disease.
- •
Graft-versus-host disease prophylaxis should include either antithymocyte globulin or alemtuzumab, and the stem cell source should be bone marrow.
- •
Exceptions to this recommendation would be recent protocols using haploidentical family donors.
- •
Haploidentical and cord blood transplants remain an experimental procedure, whereas HLA-identical donors and a well-matched (8/8) unrelated donor can be considered standard of care.
Human leukocyte antigen–identical sibling transplants
Who Is a Candidate
Patients with acquired severe aplastic anemia (SAA) should be typed for human leukocyte antigen (HLA) at diagnosis, together with their siblings: if an HLA-identical sibling is identified, and if the patient is younger than 50 years, an allogeneic bone marrow transplant (BMT) is currently the recommended first-line treatment. Usually in patients older than 50 years, immunosuppressive therapy (IST) is given up front, because mortality increases with increasing age of patients. In a study conducted by the European Group for Blood and Marrow Transplantation (EBMT), a 5-year survival advantage was seen for young patients with very low neutrophil counts (<0.2 × 10 9 /L) undergoing first-line BMT ; the opposite was true for a 50-year-old patient with a neutrophil count greater than 0.5 × 10 9 /L, in whom the survival advantage was seen for first-line IST.
- •
Young patients with low neutrophil counts are best candidates for a first-line allogeneic transplant if an HLA-identical sibling is available. Above the age of 40, one course of immunosuppressive therapy with antithymocyte globulin (ATG) and cyclosporine may be given, also in the presence of an identical sibling.
Human Leukocyte Antigen–Identical Sibling Transplants and Stem Cell Source
Bone marrow (BM) was the only source of stem cells in the early 1970s. Cord blood (CB) and peripheral blood (PB) arrived in the late 1980s and early 1990s. PB in particular has become very popular, owing to the common belief that PB grafts are safer on the donor side and improve survival, by preventing relapse, on the patient side. Both these ideas are incorrect: from the donor’s perspective, the largest study in the literature (51,024 donations) shows significantly greater risk of severe adverse events (SAE) for PB donations (25 vs 12 for BM, P <.05), and a higher risk (though not significant) of death, with 4 deaths in 23,254 PB donations and 1 death in 27,770 BM donations. Regarding leukemia relapse, a recent randomized study has shown no difference in relapse in patients with leukemia receiving BM or PB. In addition, PB grafts increase significantly the risk of extensive chronic graft-versus-host disease (cGvHD), and cGvHD after PB transplants is less responsive to treatment. Despite these data, PB grafts also have been largely used in patients with acquired SAA in whom leukemia relapse is not an issue, mainly with the hypothesis that PB grafts would solve the problem of rejection, especially in sensitized patients. In the period 2001 through 2010 the proportion of patients in the EBMT Registry receiving PB was 40% in siblings and 48% in unrelated donor grafts (Bacigalupo A, 2014, unpublished data), and it was therefore important to assess the outcome of BM versus PB.
A first combined EBMT/Center for International Blood and Marrow Transplant Research (CIBMTR) analysis has shown a significant survival advantage for BM grafts in young patients (<20 years of age) but not in older patients (≥20 years of age): this study also confirmed the increased risk of cGvHD for recipients of PB grafts. In 2012 The EBMT published a second analysis on a much larger number of patients (N = 1886) who received a first matched sibling transplant between 1999 and 2009, with BM (n = 1163) or PB (n = 723) as a stem cell source. The conclusions of this study were unequivocal: PB grafts from HLA-identical siblings, when compared with BM grafts, were associated with a greater risk of acute grade II to IV graft-versus-host disease (GvHD) (17% vs 11%, P <.001) and a greater risk of cGvHD (22% vs 11, P <.001). The survival disadvantage for PB in comparison with BM was significant in patients aged 1 to 19 years (76% vs 90%, P <.00001) and in those 20 years and older (64% vs 74%, P = .001); this was true also for those older than 50 years (39% for PB vs 69% for BM, P = .01).
In an additional study comparing 3 different stem cell sources, namely BM, PB, and granulocyte colony–stimulating factor (G-CSF)–mobilized BM, acute and chronic GvHD were again higher in PB graft recipients, and there was no advantage for G-CSF–mobilized BM in terms of engraftment and survival.
- •
BM should therefore be the stem source of choice for HLA-identical stem cell transplants in patients with acquired aplastic anemia. The use of PB is associated with worse survival and more cGvHD.
Conditioning Regimen
Cyclophosphamide
The standard of care for sibling BMT remains the original Seattle protocol, with cyclophosphamide (CY) 50 mg/kg/d on each of 4 days, and ATG (CY-ATG). Results of this conditioning regimen are well established, with current survival ranging from 65% to 95%, according to patient age. This conditioning regimen is associated with a very low incidence of second tumors and preservation of fertility.
Fludarabine
In some centers the CY200 regimen is considered too weak for highly sensitized patients, namely patients who are refractory to platelet transfusions: in one study 38 sensitized SAA patients, with a median age of 20 years (range 14–36 years), underwent BMT with a conditioning regimen including fludarabine (FLU) 30 mg/m 2 /d for 3 days (days −9, −8, and −7) and CY 50 mg/kg/d for 4 days (days −5, −4, −3, and −2). GvHD prophylaxis consisted of cyclosporine A and short-course methotrexate (CyA-MTX). Engraftment was observed in all patients after transplantation: 25 of the 27 patients with available chimeric studies at day 180 maintained donor chimerism. The risk of grade II to IV acute GvHD was 11% and of extensive cGvHD, 25%. Graft rejection with relapse of aplasia was observed in 1 patient. The overall survival (OS) for the whole group was 79%. In this particular case, the combination CY-ATG was changed to CY-FLU, with the same large dose of CY, which delivered good results in terms of engraftment.
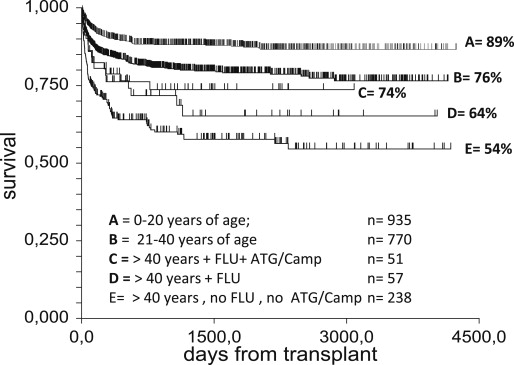
An additional problem to patient sensitization is the strong effect of age seen with the CY-ATG conditioning. For this reason, FLU-based regimens have been also introduced in older adults. In a retrospective EBMT study, patients older than 30 receiving FLU-based regimens were compared with a matched paired group of patients conditioned with CY 200 mg/kg over the same period of time (1998–2007): patients conditioned with FLU had a higher probability of OS than the control group ( P = .04) when adjusting for recipient’s age, possibly related to a trend toward a reduced incidence of graft failure in patients receiving FLU (0% vs 11%, P = .09), whereas no difference was observed regarding incidence of GvHD. In a more recent analysis of the EBMT (Bacigalupo A, 2014, unpublished data), the effect of age was combined with conditioning regimen in transplants from HLA-identical siblings, in the period 2001 through 2010 ( Fig. 1 ): survival of patients aged 1 to 20 years was 89% (group A, n = 935); survival of those aged 21 to 40 was 76% (group B, n = 770); survival of patients older than 40 receiving a FLU-based regimen and ATG or alemtuzumab (CAMP) in the conditioning regimen was 74% (group C, n = 51); survival of patients older than 40 receiving a FLU-based regimen, but no ATG and no CAMP, was 64% (group D, n = 57); and survival of those older than 40, not receiving a FLU-based regimen, and no ATG and no CAMP, was 54% (group E, n = 238). This study suggests that survival can improve in patients older than 40 years if appropriate modifications are made in the conditioning regimen.
Irradiation
Irradiation, in the form of either total lymphoid irradiation (TLI) or total body irradiation (TBI), is currently rarely used in patients receiving an HLA sibling transplant: the high risk of malignancies in SAA patients exposed to radiation during conditioning has discouraged centers from adopting this policy, although exceptions still exist.
Antithymocyte globulin and alemtuzumab
The role of in vivo T-cell depletion with ATG or CAMP for BMT in HLA-identical siblings has been disputed: a randomized trial failed to show a survival advantage for patients receiving ATG in the conditioning regimen when compared with controls. The EBMT retrospective study, on much larger number of patients, has shown a clear advantage for ATG-treated patients. A recent United Kingdom study has compared the use of ATG with the use of CAMP for in vivo T-cell depletion. One hundred patients received CAMP and 55 patients ATG-based regimens; the donor was a matched sibling in 56%, a matched unrelated donor in 39%, and other related or mismatched unrelated donor in 5% of patients. Engraftment failure occurred in 9% of the CAMP group and 11% of the ATG group. The outcome was similar for sibling BMT using CAMP or ATG (91% vs 85%, respectively; P = .562). A lower risk of cGvHD was observed in the CAMP group (11% vs 26%, P = .031). Also in this study the use of BM as stem cell source was associated with better OS and event-free survival, and less acute GvHD and cGvHD.
An additional way is to use “CAMP in the bag,” producing ex vivo T-cell depletion: the method is probably not widespread, but may lead to significant protection against chronic and acute GvHD.
- •
The standard conditioning regimen for patients with acquired SAA undergoing an HLA-identical sibling transplant remains CY200 + ATG or CAMP. In vivo T-cell depletion with ATG or CAMP seems particularly important in patients older than 40 years. FLU-based regimens may be used in older patients. Irradiation should probably be avoided in HLA-identical sibling transplants.
Engraftment and Donor Chimerism
Engraftment and graft failure have been a major issue in HLA-identical sibling transplants for acquired SAA. The use of chimerism studies, using short tandem repeat (STR) polymerase, has been a major advance in our understanding of how donor/recipient myeloid and lymphoid cells interact. In a study on 94 SAA transplants, patients were classified as (1) complete donor chimeras (n = 43%), (2) transient mixed chimeras (n = 16%), (3) stable mixed chimeras (n = 20%), (4) progressive mixed chimeras (n = 15%), and (5) early graft rejection (5%). This study showed that mixed chimerism occurs in a large proportion of patients and that the kinetics of sequential chimerism is a predictor of outcome. Treatment of mixed chimerism has not been standardized: mixed chimerism associated with declining PB counts may be treated with low doses of donor lymphocyte infusions (DLI) while maintaining GvHD prophylaxis with cyclosporine. Unfortunately there are no reports on this approach, and the use of DLI for mixed chimerism continues on a patient-to-patient basis. The author’s group has treated 9 SAA patients, with a total of 42 DLI for mixed chimerism: 6 patients achieved complete donor chimerism (100%) on BM cells and 4 on CD3 + cells; 2 patients died, 1 of rejection and 1 of GvHD; and 7 patients survive 3 to 10 years after DLI.
- •
Serial tests of donor/recipient chimerism by STR technology should be performed after transplantation in SAA, on unfractionated BM cells and possibly on selected CD3 + PB T cells. DLI, given together with cyclosporine, is effective in converting most of these patients to complete donor chimerism. Caution must be exercised with DLI dosing because of the potential risk of lethal GvHD.
The Age Effect
Determining the upper age limit for eligibility to transplant in aplastic anemia is a difficult conundrum. A strong age effect has always been reported, and still remains a major predictor of survival. As already shown herein, modifications of conditioning regimens and GvHD prophylaxis with either ATG or CAMP may improve survival in the older patient population. It would seem reasonable to restrict transplantation from identical siblings to SAA patients who are younger than 60 years. There are always exceptions to the rule, but one may wish to consider that mortality in SAA is high in older patients, probably more so than in leukemia patients of the same age.
Human leukocyte antigen–identical sibling transplants
Who Is a Candidate
Patients with acquired severe aplastic anemia (SAA) should be typed for human leukocyte antigen (HLA) at diagnosis, together with their siblings: if an HLA-identical sibling is identified, and if the patient is younger than 50 years, an allogeneic bone marrow transplant (BMT) is currently the recommended first-line treatment. Usually in patients older than 50 years, immunosuppressive therapy (IST) is given up front, because mortality increases with increasing age of patients. In a study conducted by the European Group for Blood and Marrow Transplantation (EBMT), a 5-year survival advantage was seen for young patients with very low neutrophil counts (<0.2 × 10 9 /L) undergoing first-line BMT ; the opposite was true for a 50-year-old patient with a neutrophil count greater than 0.5 × 10 9 /L, in whom the survival advantage was seen for first-line IST.
- •
Young patients with low neutrophil counts are best candidates for a first-line allogeneic transplant if an HLA-identical sibling is available. Above the age of 40, one course of immunosuppressive therapy with antithymocyte globulin (ATG) and cyclosporine may be given, also in the presence of an identical sibling.
Human Leukocyte Antigen–Identical Sibling Transplants and Stem Cell Source
Bone marrow (BM) was the only source of stem cells in the early 1970s. Cord blood (CB) and peripheral blood (PB) arrived in the late 1980s and early 1990s. PB in particular has become very popular, owing to the common belief that PB grafts are safer on the donor side and improve survival, by preventing relapse, on the patient side. Both these ideas are incorrect: from the donor’s perspective, the largest study in the literature (51,024 donations) shows significantly greater risk of severe adverse events (SAE) for PB donations (25 vs 12 for BM, P <.05), and a higher risk (though not significant) of death, with 4 deaths in 23,254 PB donations and 1 death in 27,770 BM donations. Regarding leukemia relapse, a recent randomized study has shown no difference in relapse in patients with leukemia receiving BM or PB. In addition, PB grafts increase significantly the risk of extensive chronic graft-versus-host disease (cGvHD), and cGvHD after PB transplants is less responsive to treatment. Despite these data, PB grafts also have been largely used in patients with acquired SAA in whom leukemia relapse is not an issue, mainly with the hypothesis that PB grafts would solve the problem of rejection, especially in sensitized patients. In the period 2001 through 2010 the proportion of patients in the EBMT Registry receiving PB was 40% in siblings and 48% in unrelated donor grafts (Bacigalupo A, 2014, unpublished data), and it was therefore important to assess the outcome of BM versus PB.
A first combined EBMT/Center for International Blood and Marrow Transplant Research (CIBMTR) analysis has shown a significant survival advantage for BM grafts in young patients (<20 years of age) but not in older patients (≥20 years of age): this study also confirmed the increased risk of cGvHD for recipients of PB grafts. In 2012 The EBMT published a second analysis on a much larger number of patients (N = 1886) who received a first matched sibling transplant between 1999 and 2009, with BM (n = 1163) or PB (n = 723) as a stem cell source. The conclusions of this study were unequivocal: PB grafts from HLA-identical siblings, when compared with BM grafts, were associated with a greater risk of acute grade II to IV graft-versus-host disease (GvHD) (17% vs 11%, P <.001) and a greater risk of cGvHD (22% vs 11, P <.001). The survival disadvantage for PB in comparison with BM was significant in patients aged 1 to 19 years (76% vs 90%, P <.00001) and in those 20 years and older (64% vs 74%, P = .001); this was true also for those older than 50 years (39% for PB vs 69% for BM, P = .01).
In an additional study comparing 3 different stem cell sources, namely BM, PB, and granulocyte colony–stimulating factor (G-CSF)–mobilized BM, acute and chronic GvHD were again higher in PB graft recipients, and there was no advantage for G-CSF–mobilized BM in terms of engraftment and survival.
- •
BM should therefore be the stem source of choice for HLA-identical stem cell transplants in patients with acquired aplastic anemia. The use of PB is associated with worse survival and more cGvHD.
Conditioning Regimen
Cyclophosphamide
The standard of care for sibling BMT remains the original Seattle protocol, with cyclophosphamide (CY) 50 mg/kg/d on each of 4 days, and ATG (CY-ATG). Results of this conditioning regimen are well established, with current survival ranging from 65% to 95%, according to patient age. This conditioning regimen is associated with a very low incidence of second tumors and preservation of fertility.
Fludarabine
In some centers the CY200 regimen is considered too weak for highly sensitized patients, namely patients who are refractory to platelet transfusions: in one study 38 sensitized SAA patients, with a median age of 20 years (range 14–36 years), underwent BMT with a conditioning regimen including fludarabine (FLU) 30 mg/m 2 /d for 3 days (days −9, −8, and −7) and CY 50 mg/kg/d for 4 days (days −5, −4, −3, and −2). GvHD prophylaxis consisted of cyclosporine A and short-course methotrexate (CyA-MTX). Engraftment was observed in all patients after transplantation: 25 of the 27 patients with available chimeric studies at day 180 maintained donor chimerism. The risk of grade II to IV acute GvHD was 11% and of extensive cGvHD, 25%. Graft rejection with relapse of aplasia was observed in 1 patient. The overall survival (OS) for the whole group was 79%. In this particular case, the combination CY-ATG was changed to CY-FLU, with the same large dose of CY, which delivered good results in terms of engraftment.
An additional problem to patient sensitization is the strong effect of age seen with the CY-ATG conditioning. For this reason, FLU-based regimens have been also introduced in older adults. In a retrospective EBMT study, patients older than 30 receiving FLU-based regimens were compared with a matched paired group of patients conditioned with CY 200 mg/kg over the same period of time (1998–2007): patients conditioned with FLU had a higher probability of OS than the control group ( P = .04) when adjusting for recipient’s age, possibly related to a trend toward a reduced incidence of graft failure in patients receiving FLU (0% vs 11%, P = .09), whereas no difference was observed regarding incidence of GvHD. In a more recent analysis of the EBMT (Bacigalupo A, 2014, unpublished data), the effect of age was combined with conditioning regimen in transplants from HLA-identical siblings, in the period 2001 through 2010 ( Fig. 1 ): survival of patients aged 1 to 20 years was 89% (group A, n = 935); survival of those aged 21 to 40 was 76% (group B, n = 770); survival of patients older than 40 receiving a FLU-based regimen and ATG or alemtuzumab (CAMP) in the conditioning regimen was 74% (group C, n = 51); survival of patients older than 40 receiving a FLU-based regimen, but no ATG and no CAMP, was 64% (group D, n = 57); and survival of those older than 40, not receiving a FLU-based regimen, and no ATG and no CAMP, was 54% (group E, n = 238). This study suggests that survival can improve in patients older than 40 years if appropriate modifications are made in the conditioning regimen.