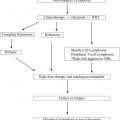
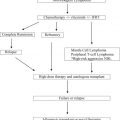
Thanks to the advent of reduced-intensity conditioning regimens and improvements in supportive care, we now have the ability to safely perform transplantations for older patients and those with comorbid illnesses. In some centers, it is not uncommon to perform autologous or even allogeneic stem cell transplants in patients as old as age 75 years. Long-term studies suggest that average health-related quality of life and functional status among survivors, including older patients, recover within a couple of years to pretransplant levels.
A major obstacle to success with allogeneic bone marrow transplant has been the frequent lack of suitable matched donors. Only one-quarter of siblings will be an HLA match, and there is only a 50% chance overall, and much lower for African Americans and ethnic minorities, of finding a matched unrelated donor. Time is also a factor; it can take months to coordinate an unrelated transplant. New findings show that HSCTs are more accessible for patients previously not considered good candidates. Since 2007, more allo-HSCT procedures have been performed using alternative donor stem cell sources, such as volunteer unrelated donors or cord blood, than related donors. RIC haploidentical-related donor or cord blood transplantations have emerged as alternatives to fill the gap for those patients who do not have matched related donor or unrelated donor. Recent data support that haploidentical HSCT can be performed safely, yield good outcomes, and greatly expand the number of patients who can be treated with stem cell transplant. In this era, a stem cell source can be found for virtually all patients who have an indication to receive allogeneic HSCT. Between the cord blood and haploidentical transplant, unrelated donor transplant, and matched sibling donor, there is nobody who needs a transplant who should not get it in this day and age, as long as they are healthy enough to undergo the transplant.
Due to the availability of novel substances and treatment strategies, the standards of care in many malignancies have changed dramatically. These new approaches include new monoclonal antibodies, immune modulatory agents, substances interfering with the BCR signaling pathway, and novel cellular therapies. The choice of HSCT versus a novel agent is one that must be gauged on a patient-by-patient basis.
A very exciting new active immunotherapy strategy is chimeric antigen receptor (CAR) T-cell therapy. CAR technology has recently emerged as a novel and promising perspective to specifically target malignant cells with precisely engineered T cells. Several clinical trials have reported impressive results with anti-CD19 CARs, in both chronic lymphocytic leukemia and acute lymphoblastic leukemia, and therapy has been investigated in other malignancies.
As there are no direct comparisons between HSCT and novel agents, general evidence-based recommendations are very difficult to make at this point. Instead, we need to understand the limitations of each approach and carefully weigh the chances and risks of each procedure on a case-by-case basis. In general, the availability of treatments, their expected benefit and side effects, and individual treatment histories and pretransplant characteristics as determined by the variety of risk score system, need to be taken into consideration. As the success of HSCT is, however, highly dependent on the remission state of the time of HSCT, it seems very desirable to focus on achieving disease control first. This can be facilitated by novel substances. As they are also well tolerated and show only moderate toxicities, they seem a good option to bridge the time until HSCT, and maybe even to postpone HSCT to a later point in the disease. How these substances should be best combined, if there is the option to completely eliminate the chemotherapy backbone from induction or second-line treatment, and whether they will have an effect on graft-versus-tumor and immunomodulation are the major focus of ongoing preclinical and clinical studies.
Indeed, the use of HSCT continues to grow each year in the United States, Europe, and around the world. In parallel with advances in other cancer treatments, HSCT has evolved rapidly in the past 2 decades in ways that may be unfamiliar to those who learned about transplant earlier in their careers. Nevertheless, continued underutilization of transplantation in patients who might otherwise benefit suggests that many of the improvements in the field may not be well-known among referring providers.
In this issue of Hematology/Oncology Clinics of North America , a multidisciplinary team has compiled an issue that details the state-of-the-art of HSCT management for commonly indicated benign and malignant hematological disease. The issue is therefore timely and at the same time unique. The contributions from acknowledged experts in the field from around the globe are covering the organizational aspects of transplant patients. This issue presents the most current knowledge about how to integrate transplantation and novel therapies in patients with benign and malignant diseases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree