Chapter 13 Shubha Allard Clinical Haematology, Barts and the London NHS Trust, NHS Blood and Transplant, London, UK Careful donor selection followed by stringent testing by the blood service is essential to ensure a safe blood supply. In developed countries, blood is processed into its component parts such as red cells, platelets, fresh frozen plasma (FFP) and cryoprecipitate and granulocytes for clinical use and not transfused as whole blood. Donated blood is a limited resource, and hospital blood transfusion practice must focus on ensuring safe and appropriate use including the use of clinical guidelines supported by education and training with regular audit of practice. Robust systems are essential for accurate patient identification throughout the transfusion process and include the final bedside check to minimize errors. Transfusion medicine must be practised within a strict regulatory framework – the European Union (EU) blood directives have been transposed into the UK law with the Blood Safety and Quality Regulations (BSQR) 2005 setting standards for quality and safety in the collection, testing, processing, storage and distribution of human blood components [1]. The regulations affect both the national blood services (called blood establishments in the BSQR) and hospital transfusion laboratories. All donors in the UK are voluntary and unpaid. They are carefully selected using a donor health questionnaire to ensure that they are safe to donate and to exclude anyone at risk of transmitting infection. Donors can give around 450 mL of whole blood three to four times a year, which is separated into red cells, platelets and plasma. These individual components can also be collected by separate component donation using apheresis [2]. The UK plasma is no longer used for fractionation for manufacture of blood products such as albumin, intravenous immunoglobulin, anti-D or factor concentrates (see succeeding text). Transfusion transmitted infection. In the UK, all donations are tested for syphilis, hepatitis B, hepatitis C, HTLV1 and HIV. The tests used and the estimated risk [3] are summarized in Table 13.1. Table 13.1 Transfusion transmitted infection in UK: Estimated risk in 2010–2013 [3]. Some donations are also tested for cytomegalovirus (CMV) antibody to help provide CMV-negative blood for particular patient groups. The Specialist Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO) in the UK has reviewed evidence [4] available and recommended that leucodepletion of all blood components (other than granulocytes) provides adequate CMV risk reduction for various patients requiring transfusion (haemopoietic stem cell transplant patients, organ transplant patients and immune-deficient patients, including those with HIV) without the requirement for CMV-seronegative components in addition. However, CMV-seronegative red cell and platelet components should be provided for intrauterine transfusions and for neonates and for pregnant women requiring repeat elective transfusions during the course of pregnancy. Donors are tested for malaria and T. cruzi antibodies when indicated by their travel history. Other discretionary tests include anti-HBc (e.g. after body piercing). Variant Creutzfeldt–Jakob disease (vCJD). To date, there have been four cases in the UK where blood transfusion may have been implicated in transmission of new variant Creutzfeldt–Jakob disease (vCJD). A further transmission of variant CJD prions was described in February 2009 in a patient with haemophilia who had received batches of factor VIII (FVIII) to which a donor who subsequently developed variant CJD had contributed plasma. The patient died of other causes but was found to have evidence of prion accumulation in his spleen. There is no blood test at present readily available for detecting prions, although there is active international research ongoing in this field. The full risk of vCJD in the UK population remains uncertain, and accordingly, the UK blood services have taken a number of precautionary measures to reduce the potential risk of transmission of prions by blood, plasma and blood products, the latter requiring fractionation of very large volumes of plasma [5]. These include: Donor blood is collected into plastic packs containing citric phosphate dextrose and then transported without delay to the blood centre for processing. All units undergo initial leucodepletion to remove white cells. Further processing (see Figure 13.1) is then undertaken to produce red cells, platelets and plasma or cryoprecipitate under stringent standards of quality control as mandated by the BSQR 2005. Figure 13.1 Processing of blood components. Source: Reproduced with permission from Handbook of Transfusion Medicine [6]. Crown Copyright. During processing, the majority of plasma is removed and replaced with an optimal additive solution comprising saline, adenine, glucose and mannitol (SAG-M). Citrate binds calcium and acts as an anticoagulant, and the glucose and adenine support red cell metabolism during storage. A standard red cell component in additive solution contains red cells (Hct 0.50–0.70 and Hb content > 40 g/U), 5–30 mL of plasma and 100 mL of SAG-M solution in a total volume of 220–340 mL [3]. Standard red cells contain no functional platelets, granulocytes or coagulation factors and are stored at 4 °C with a shelf life of 35 days. An adult therapeutic dose (ATD) or 1 U of platelets can either be produced by single-donor apheresis or by centrifugation of whole blood followed by separation and pooling of the platelet-rich layer from four donations suspended in plasma. Platelets can be stored for 5 days at 20–24 °C with constant agitation. Bacterial testing of platelets prior to release now introduced by some of the blood services such as NHS Blood and Transplant can reduce the risk of bacteriological contamination and with an extension of the shelf life of platelet units to 7 days. Platelet components must not be placed in a refrigerator. Fresh frozen plasma is a source of coagulation factors and is produced by separation and freezing of plasma at –30 °C. Single donation units, sourced from non-UK plasma and treated with methylene blue to reduce microbial activity, are indicated for all children born after 1996. Solvent–detergent plasma is prepared commercially from pools of 300–5000 plasma donations that have been sourced from non-UK donors and treated with solvent and detergent to reduce the risk of viral transmission (Table 13.2). Table 13.2 Comparisons of standard fresh frozen plasma (FFP) with solvent–detergent FFP. Cryoprecipitate is prepared by undertaking controlled thawing of frozen plasma to precipitate high-molecular-weight proteins including FVIII, von Willebrand factor and fibrinogen. Cryoprecipitate consists of the cryoglobulin fraction of plasma containing the major portion of FVIII and fibrinogen. It is obtained by thawing a single donation of FFP at 4 °C ± 2 °C. The cryoprecipitate is then rapidly frozen to −30°C. In the UK, it is available as pools of 5 U. The chief executive of each hospital with a transfusion laboratory needs to submit a formal annual statement of compliance to the Medicines and Healthcare products Regulatory Agency (MHRA). Hospital transfusion laboratories can be inspected by the MHRA [5] and in the event of significant deficiencies can be given the order to cease and desist from activities. The key requirements for hospital transfusion laboratories include: Hospital transfusion laboratories undertaking any processing activities such as irradiation must have a licence from the MHRA indicating blood establishment status. A series of three Better Blood Transfusion
Blood Components and Their Contents
Introduction
Blood transfusion and the regulatory framework
Donor selection and testing
Microbiological testing of donor blood
Test
Testing introduced
Examples of testing methods used
Approximate risk of infection
Syphilis
1940s
Antibody
Hepatitis B
1970/2009 onwards
Surface antigen/(HBsAg)/Nucleic acid testing
1 in 1million
HIV
1985/2001 onwards
Antibody/nucleic acid testing
1 in 7 million
HCV
1991/1999 onwards
Antibody/nucleic acid testing
1 in 29 million
HTLV
2002
Antibody
Processing of blood
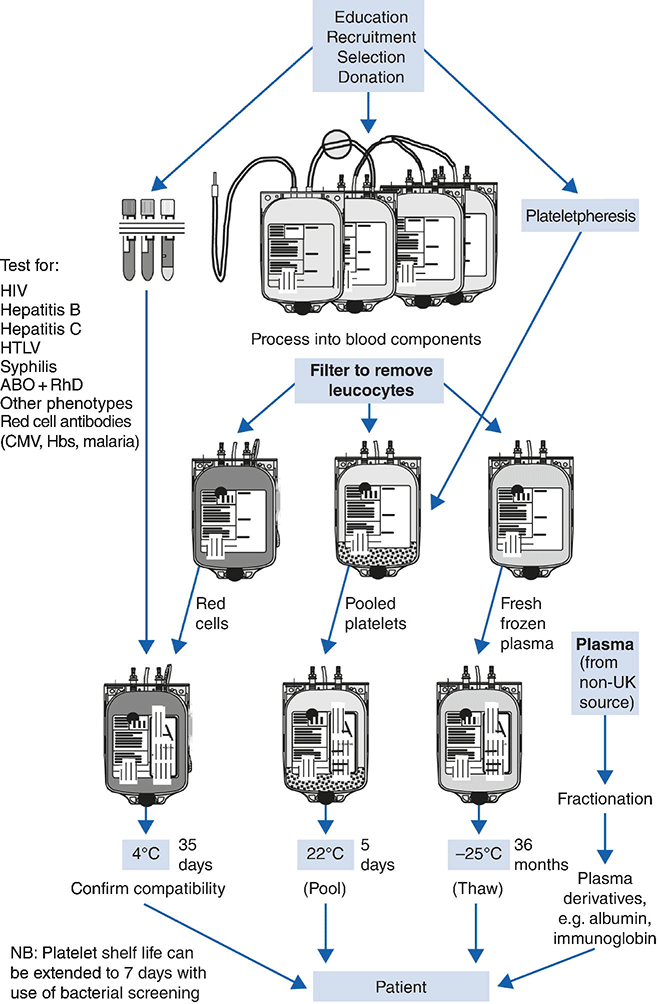
Red cells in additive solution
Platelets
Fresh frozen plasma
Standard adult FFP
SD-FFP
Source
Single donor for each unit of FFP
60–1500 donors contributing to plasma pool
Supplier
Supplied by blood service
Supplied by commercial manufacturer
Volume
250–300 mL
200 mL
Product licence
Not required
Licensed, batch product
Thawing needed
Yes
Yes
Coagulation factor content
Variable between units: 75% of units have FVIII >0.7 IU/mL
Constant within batch: all factors >0.5 IU/mL
Viral inactivation
No
Yes
Cryoprecipitate
Clinical and laboratory transfusion practice
Blood Safety and Quality Regulations: Impact on hospital transfusion practice
Better Blood Transfusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree