BLOOD AND BLOOD COMPONENT THERAPY—21ST CENTURY CONSIDERATIONS
Opportunities are endless to transform and improve the administration of blood and blood components. With more than 5 million patients requiring these products per year, transfusion therapy needs to be safe and address innovative therapeutics while being cost-effective and efficient. Depending on the geographical location, blood donation continues to be in a high demand as the supply continues to fluctuate due to unexpected events such as mass casualties, hurricanes, and earthquakes (
U.S. Department of Health and Human Services [HHS], 2011). Maintaining a 3-day supply of blood remains imperative to meet the transfusion demands of blood and blood components. With over 15 million transfusions provided in 2008, it is essential to instill trust in the blood collection and transfusion process for the recipient (HHS, 2011).
Regulatory bodies such as The Joint Commission (TJC) have focused efforts on safe transfusion practices through their National Patient Safety Goals (
TJC, 2013). Blood transfused to the wrong patient is a major cause of patient complications; therefore, specific attention should focus on improving the process of blood verification and administration (
American Association of Blood Banks [AABB], 2011). Safe transfusions are possible through strictly adhering to regulations and continually improving blood component testing.
One of the most important steps is identification of the patient for each procedure in the transfusion process. As a critical component to reduce adverse effects, correct identification has been at the forefront of discussions with the AABB, U.S. Food and Drug Administration (FDA), as well as donation and transfusion services. A current mandate is that patients must be identified using two identifiers, such as the patient name and date of birth (
TJC, 2013). Accurate identification of the patient and verification of the correct blood component allow the correct patient to receive the correct blood. Realizing it was imperative to correctly identify the right patient; multiple requirements have been set forth since 2004. The FDA instituted a “bar code rule” in 2004 stating that some drug and all blood-related packaging must provide a bar code label. In the same statement, the FDA required machine-readable information. The intention was to prepare for technology-related identification of patients and blood components such as machine-readable bar codes. Recognizing the difficulty of implementing such a system with limited funds, the FDA began revisiting ideas to facilitate its role in patient safety. The FDA acknowledged both the positive and negative aspects of implementing such a system. Although in a 20-year span, bar coding would prevent nearly 500,000 adverse events and transfusion errors, the cost would total $93 billion (HHS, 2013).
Another technological advancement for safer blood transfusion practices is
radiofrequency identification devices (RFID). A small chip can be implanted into wristbands, laboratory tubes, and blood component bags. The transmitter functions over radio waves and alerts the transfusionist of incompatibility instead of relying on the precise optical reading requirements of the bar coding technology (
Brooks, 2008; Davis, Geiger, Gutierrez,
Heaser, & Veeramani, 2009). Current research is focused on feasibility and cost-effectiveness of RFID for the entire transfusion process (
Hohberger, Davis, Briggs, Gutierrez, & Veeramani, 2012).
Although enhanced technology assists in positive patient identification, there was a need to make blood and blood component labeling universal and reduce errors related to misread labels. In 2008, all accredited facilities implemented a standardized system called the International Society of Blood Transfusion (ISBT 128) bar code labels. Continued exploration of technology and processes are needed to make transfusion therapy safer and efficient at the bedside.
Nurses will also need to maintain their knowledge and competence with each new blood and blood component product. Recent advances in transfusion therapy such as hematopoietic growth factors, blood substitutes, cord blood, gene therapy, and cellular engineering are improving patient outcomes. Nurses are the last checkpoint to ensure safe administration of transfusion therapy. Along with the patient, nurses are the first to identify possible transfusion reactions and can intervene appropriately. Nursing care is the surveillance, assessment, problem identification, and intervention that keep patients safe.
The future of health care leans on interprofessional collaboration to provide a safe patient environment. Through interprofessional interactions, nurses must encourage patient-centered care (
Institute of Medicine, 2011). As current and future research continues to advance transfusion therapy, nurses must understand the importance of their role in including patients in their care. As advocates for patient safety and improving quality of care, nurses must work closely with patients requiring one or more transfusions. Nurses need to be aware of these innovations to explain how blood is made safe and to be alert to the potential for the patient’s reactions to a blood-processing methodology. By understanding the patient’s concerns related to blood transfusions, nurses can assist by evaluating patient education materials and by involving patients in the decision-making process.
Nurses are integral health care partners in transfusion therapy. Along with providers in their own facilities, nurses work with many regulatory agencies to improve care. Some of these agencies in the United States include the Centers for Disease Control and Prevention (CDC), the FDA, the Occupational Safety and Health Administration, the AABB, the Clinical Laboratory Improvement Act, and TJC. The AABB sets standards that represent accepted performance guidelines for the provision of safe blood banking, transfusion, transplantation, and work environments for personnel. Professional organizations also assist in the maintenance of standards, both for professionals and health care agencies, such as the American Nurses Association (ANA), the American Association of Critical-Care Nurses (AACN), the Infusion Nurses Society (INS), and the Oncology Nursing Society (ONS). Adhering to the standards of these agencies instills confidence in providers and patients that care is current and policies and procedures are followed. The future for transfusion therapy is filled with opportunities. Nurses can provide the safest therapy for patients and minimize risks when working collaboratively with all health care colleagues toward this common goal.
BASIC IMMUNOHEMATOLOGY
Immunohematology is the science that deals with antigens of the blood and their antibodies. Antigens (also called agglutinogens because they can cause blood cell agglutination, or clumping) are substances capable of stimulating the production of antibodies in response to their presence on the blood cell. An antibody is a protein in the plasma that reacts with this specific antigen. People who have been exposed to the red blood cells (RBCs) or leukocytes of other people through transfusion, transplantation, or pregnancy (maternal-fetal hemorrhage) may produce antibodies to the antigens carried on those foreign cells.
Antibodies are named for the antigen that stimulated their formation and with which they react. For example, an antibody against the Rh, or “D” antigen, formed after the transfusion of an Rh (D)-positive unit to an Rh (D)-negative recipient, is called anti-D. Antibodies formed in response to exposure to foreign antigens are called immune antibodies.
Numerous antigens have been discovered on human blood cells, some more commonly encountered, such as Kell, Duffy, and Kidd. There are, however, hundreds of antigens that are rarer. Most of these are weak and of minimal importance in transfusion. Leukocytes also carry antigens on their surfaces, the most important being human leukocyte antigens (HLA). The groups most likely to cause transfusion reactions are the ABO and Rh systems of antigens.
The ABO system, discovered by Karl Landsteiner in 1901, is the most important group of antigens for transfusion as well as for transplantation. The synthesis of these antigens, which are located on the surface of RBCs, is under the control of the A, B, and O genes. There are four ABO blood types: In the presence of the A gene, a person makes A antigen and is classified as group A; group B people have B antigens on their RBCs; group AB people have both; and group O people have neither. These antigens, inherited genetically, determine the person’s blood group.
Some blood group antibodies are formed without exposure to
allogeneic (from another person) RBCs. The most important of these so-called naturally occurring antibodies, or isoagglutinins, are anti-A and anti-B. During the first few months of life, infants make antibodies to whichever of the ABO antigens they lack. For example, a group A infant makes anti-B (
Table 17-1). These isoagglutinins can produce rapid hemolysis of RBCs with the corresponding antigen, forming the basis of ABO incompatibility.
The
Rh system, discovered in 1940 by Stetson and Levine, is the second most clinically important system for transfusion. The Rh system is so-called because of the discovery of this substance in the RBCs of the Rhesus monkey. The antigens of the human Rh system are of great
clinical significance because they often are responsible for transfusion incompatibility and hemolytic disease of the newborn (HDN). Approximately 85% of the white population has the D antigen on their RBCs and are classified as Rh positive, and 92% of African Americans are Rh positive (
AABB, 2010). In the remainder of the population, the D antigen is missing; these people are classified as Rh negative and can readily form anti-D after exposure to the antigen. Severe hemolytic reactions and HDN result from this antigen-antibody reaction.
Mechanism of Immune Response
When a patient is transfused with allogeneic blood bearing foreign (i.e., nonself) antigens, the immune system may respond by producing antibodies. Phagocytic cells called
macrophages play an important role in this response by capturing and processing foreign antigens. The macrophages then present the processed antigens to other members of the immune system, called
T and
B lymphocytes, which begin to produce antibodies specific for the antigens presented. Stimulated by the foreign antigen, the B cell swells, changes its internal structure, and differentiates into plasma cells or memory B cells that produce the antibodies to bind and aggregate foreign cells and ensure their removal (
Sommer, 2009). Each plasma cell then produces large quantities of antibodies specific to the particular antigen as genetically programmed into the cell. The cells secrete the antibodies into a variety of body fluids, including the blood plasma. The T lymphocytes act as helpers or suppressors of B-lymphocyte function. The intertwined relationship between B and T lymphocytes regulates the type and sensitivity of the immune response.
Mechanism of Red Blood Cell Destruction
Two mechanisms of RBC destruction are known: intravascular hemolysis and extravascular hemolysis. Intravascular hemolysis occurs within the vascular compartment. This mode of RBC destruction results from the sequential binding of an antibody to a foreign antigen on the surface of a RBC. This reaction then activates the complement system. When the complement system cascade is triggered, a resulting lytic complex forms on the RBC membrane, which leads to rupture of the RBC and the rapid release of free hemoglobin into the intravascular compartment overwhelming the system. The most frequent cause of this type of RBC destruction is transfusion of ABO-incompatible blood. Mostly immunoglobulin (IgM) antibodies, anti-A and anti-B, are capable of engaging the complement, coagulation, and kinin systems when they bind with their corresponding antigens. The consequences of this type of hemolysis are usually immediate, severe, and often fatal.
The other mechanism of RBC destruction, extravascular hemolysis, is more commonly seen as a result of incompatibilities in blood group systems other than the ABO. In contrast to anti-A and anti-B, antibodies to these other blood group antigens are usually immunoglobulin G (IgG) and bind to RBCs with the corresponding antigen. Phagocytic cells of the reticuloendothelial system, particularly in the spleen, bind the IgG-coated RBCs, ingest them, and destroy them extracellularly—hence the term
extravascular hemolysis. These IgG antibodies also may fix some amount of complement, but not enough to lyse the RBCs. Instead, the phagocytic cells of the liver, the Kupffer cells, clear the complement-coated RBCs from the circulation and destroy them intracellularly. Although the rapid clearance of red cells can induce systemic inflammation and cytokine storm, the consequences of extravascular hemolysis are less severe than those of intravascular hemolysis. Extravascular
hemolysis is recognizable by a drop in the hematocrit, an increase in the bilirubin level, and perhaps the clinical signs of fever or malaise.
Blood Group Systems
As discussed, the ABO system is the only system in which antibodies (anti-A and anti-B) are consistently and predictably present in the sera of people whose RBCs lack the corresponding antigen(s). The ABO system is the foundation of pretransfusion testing and compatibility.
Antibodies to the Rh (D) antigen are not present unless a person has been exposed to D-positive RBCs, through either transfusion or pregnancy. Because of the ease with which antibody D is made, grouping is done on all donors and recipients to ensure that D-negative recipients receive D-negative blood, except for reasonable qualifying circumstances. D-positive recipients may receive either D-positive or D-negative blood. People who develop Rh antibodies have detectable levels for many years. If undetected, subsequent exposure may lead to a secondary immune response. Occasionally, weak variants of the Rh (D) antigen are found, which are identified by special serologic tests. These people are termed weak D or Du positive, but they are considered Rh positive both as donors and recipients.
Other blood group systems have been defined on the basis of the cell’s reactions to antigens. Antibodies to antigens in the Kell, Duffy, and Kidd systems are encountered frequently enough to be clinically important. Antibodies to the blood group antigens in most other systems are found so infrequently that they do not cause everyday problems. When present, these antibodies may produce hemolytic reactions. Once demonstrated, precautions must be taken to ensure that the patient receives compatible blood lacking the corresponding antigen. When difficulty arises in cross-matching or when a transfusion reaction occurs, these systems acquire special significance. Today, systems to identify, validate, and ensure ABO and Rh groupings are set up on computer databases. Confirmatory testing must be in place according to AABB guidelines.
PRETRANSFUSION TESTING, BLOOD DONATION, AND BLOOD PRESERVATION
The Donor
Consistent with American Red Cross (ARC) guidelines, a donor may donate whole blood or red cells no more than every 8 weeks. A double red cell donation may be made no sooner than 16 weeks (
ARC, 2013). Platelets may be donated every 7 days but donation frequency cannot exceed a maximum of 24 times per year (
ARC, 2013). A donation shall be no more than 10.5 mL/kg body weight. In addition to ABO and Rh group determination and a screen for unexpected antibodies, several other tests are performed on donors, including a physiologic health assessment, questionnaire, and blood sample. These tests must be negative for the antibodies identified (
Box 17-1) before any blood or component is released for patient use.
The Recipient
Tests performed on the intended transfusion recipient include ABO and Rh group determination and screening for unexpected antibodies. Before the administration of whole blood or RBC components, a major cross-match (combining donor RBCs and recipient serum or plasma) is
performed to detect serologic incompatibility. The AABB requires that if a patient has been transfused in the preceding 3 months with blood or a blood component containing RBCs, has been pregnant within the preceding 3 months, or the history is uncertain or unavailable, the sample must be obtained from the patient within 3 days of the scheduled transfusion (
AABB, 2010). This requirement is made to rule out the possibility of missing a newly formed antibody.
In situations in which delaying the provision of blood may jeopardize life, blood may be issued before completion of routine tests according to AABB standards (
AABB, 2011). Recipients whose ABO group is not known must receive group O RBCs. Physicians/licensed independent practitioners (LIPs) must indicate in the record that the clinical condition is sufficiently urgent to release blood before the completion of compatibility testing. The tag or label must conspicuously indicate that compatibility testing is not completed, and standard compatibility tests should be completed as promptly as possible. Recipients whose ABO group has been determined by the transfusing facility without reliance on previous records may receive ABO group-specific whole blood or ABO group-compatible RBC components before other compatibility tests have been completed (
Box 17-1).
Directed Donations
The risk of transfusion-transmitted diseases has generated programs whereby prospective patients may designate their own blood donors. Blood centers and hospitals have increased the availability of this program, known as
directed donation, for the public. Each donor enters the same process as any other, but the blood is labeled for a specific recipient provided
screening tests are appropriate and the unit is compatible. Units are specifically labeled for the intended recipient; however, the component may be used for allogeneic donation if not needed by the individual for whom the donation was intended.
The primary benefit of this program may be to reduce the fear of disease transmission in recipients who feel reassured knowing who their donors are. If the donor is a blood relative of the recipient, the cellular components must be irradiated to prevent graft versus host disease (GVHD) (
AABB, 2010). These programs must address cost-effectiveness, staffing, quality assessment measures, and legal considerations.
Autologous Donation
Autologous transfusion is the collection, filtration, and reinfusion of a person’s own blood; thus, the donor and recipient are the same individual. There are several types of autologous donation, all of which eliminate the risk of disease transmission as well as alloimmunization, although other mechanical and nonhemolytic reactions can occur. Autologous transfusion takes on several different meanings and methods and is accepted practice for appropriate candidates in a specific setting based on the patient’s clinical condition.
Autologous transfusion is a conservative approach. In the 1980s and early 1990s, the acquired immunodeficiency syndrome (AIDS) epidemic and increased awareness of blood-borne disease transmission created interest and demand for autologous blood collection. This interest, however, decreased to only 1.5% of the total donations in 2008 (
AABB, 2011) because of improved donor screening and public perception of a safe blood supply. As the possibility of new transfusion-transmitted diseases is identified or significant reductions in the blood supply occur, the demand for autologous donations may rise (
AABB, 2011).
Although receiving one’s own blood is the safest transfusion, careful administration of autologous blood is still required. To avoid any errors, those administering the blood must verify all aspects of the component, including proper identification. Verification procedures should be identical to those for giving homologous transfusions. All products of autologous donation must be labeled “For Autologous Use Only.”
The AABB endorses all modalities of blood conservation, including the goals of autologous transfusions. Autologous transfusion can be accomplished by preoperative donation, acute normovolemic hemodilution, intraoperative salvage, and postoperative blood salvage.
PREOPERATIVE AUTOLOGOUS DONATION
This technique is best used when a patient is planning an elective surgical procedure that normally would cause the loss of a significant number of units of blood. The patient may plan periodic visits to a donor center for phlebotomies to store his or her own blood for later use. Consideration should be made for patients donating preoperatively to include receiving recombinant erythropoietin to rebuild red cell mass prior to surgery and decrease the likelihood of receiving perioperative transfusion.
ACUTE NORMOVOLEMIC HEMODILUTION
In this procedure, the patient’s blood is collected, stored at room temperature for up to 8 hours in the operating room, and returned to the patient at the time of blood loss. Deterioration of platelets and coagulation factors is minimal in this time frame. To maintain
plasma volume, the patient receives infusions of a crystalloid or colloid solution. The benefit of this technique is that it reduces the patient’s hematocrit by dilution before surgical blood loss so that the patient loses a smaller number of red cells. Frequently used in cardiac surgery, hemodilution may be limited by the patient’s blood volume or other hemodynamic considerations. Because the hematocrit is reduced, patients with lung disease or hypooxygenation require close monitoring.
INTRAOPERATIVE BLOOD COLLECTION
This frequently used method of autologous transfusion involves recovering blood during surgical procedures associated with significant blood loss from clean wounds. Blood is aspirated from the operative site, washed using cell-washing machines, and reinfused to the patient. The process may be performed completely in the operating room. It is most frequently used in cardiovascular, thoracic, orthopedic, neurologic, and hepatic surgery, including transplantations. The technique makes large volumes of blood immediately available when bleeding occurs in a clean operative procedure. The procedure is contraindicated in patients with malignancy or infection because reinfusion of contaminated cells may disseminate the tumor cells or infectious organism.
Sterile technique is mandated to prevent infectious agents from entering the system. Initial equipment and personnel costs can be high, but improved medical care outweighs the disadvantages. Institutions that provide intraoperative blood collection require policies, processes, and procedures that address day-to-day operations.
POSTOPERATIVE BLOOD COLLECTION
This technique involves salvaging blood shed from surgical drains after cardiac, trauma, orthopedic, and plastic surgery. Other clinical conditions also may be appropriate. The advantage of this technique is that it can be done for planned surgery or emergency operations and trauma. Postoperative shed blood is collected through special equipment for reinfusion. If the blood in the sterile canister is not reinfused within 6 hours, it must be discarded. This technique is safe, simple, and cost-effective. Combined with other methods of autologous transfusions described, the goal of avoiding homologous blood transfusions is nearer.
Blood Preservation
Blood is routinely collected in sterile plastic bags that contain different anticoagulant-preservative solutions. Whole blood may be collected in anticoagulant citrate-phosphate-dextrose solution (CPD), citrate-phosphate-dextrose-dextrose solution (CP2D), or anticoagulant citrate-phosphate-dextrose-adenine solution (CPDA-1). Since patients may suffer an adverse reaction to an anticoagulant-preservative, it is important to be knowledgeable of chemicals used in the solutions. Citrate is an anticoagulant that binds with free calcium in the donor’s plasma. Since blood requires calcium to clot, the presence of citrate inhibits the coagulation cascade. Phosphate buffers the pH, preventing a decrease in 2,3-diphosphoglycerate (2,3-DPG), thus facilitating the oxygen-carrying capacity of the blood. Dextrose (glucose) prolongs the life of the RBCs by providing a nutrient source. Blood cells may be stored in CPD for 21 days at 1°C to 6°C. CPDA-1 contains adenine, which helps the RBCs
synthesize adenosine triphosphate (ATP) during storage and lengthens the shelf life of the blood to 35 days. The RBC depends on ATP to maintain cell surface ion pumps.
The duration of time for storage is based on the standard that a minimum of 75% of the transfused RBCs (stored for 35 days) must be present in the bloodstream of the recipient 24 hours after the transfusion. Providing a nutrient biochemical balance to RBCs during storage is important to maintain viability and function of the components in the blood and to prevent physical changes. To minimize bacterial proliferation, specific temperatures are maintained.
A second group of anticoagulant-preservative systems labeled as additive solutions is similar to CPDA-1. They differ, however, in that once the platelet-rich plasma is removed from the unit within 8 hours, an additive solution of 100 mL containing saline, dextrose, and adenine is added to the packed RBCs. These additive systems permit storage for up to 42 days. FDA-approved solutions include AS-1 (Adsol), AS-3 (Nutricel), and AS-5 (Optisol).
WHOLE BLOOD
Whole blood contains RBCs, white blood cells, platelets, plasma (blood proteins, antibodies, water, and waste), and electrolytes. The usual volume is 450 to 550 mL/unit. Transfusions of whole blood are rare and indicated only in acute, massive blood loss for the purpose of expanding blood volume and increasing the oxygen-carrying capacity. If possible, blood loss should be managed with other blood components, crystalloids, or colloidal solutions.
Administration of whole blood subjects the patient to the possibility of complications such as fluid volume excess, especially in patients with a compromised cardiac status. In addition, after 24 hours of storage, cell breakdown can result in elevated potassium levels; the formation of microaggregates, nonviable platelets, and granulocytes; and decreased levels of the clotting factors, factor V and factor VIII. In patients with a severely damaged liver, massive whole-blood transfusions can result in a calcium deficit owing to the presence of citrate anticoagulant. Monitoring the patient’s potassium level before and after the transfusion is important. Hemoglobin and hematocrit levels may be inaccurate owing to fluid shifts during active bleeding. Whole blood transfusion must be ABO
identical with that of the recipient because of the presence of antibodies. One unit of whole blood increases hemoglobin by about 1 g/dL and hematocrit by about 3 to 4 percentage points (
American Association of Blood Banks, American Red Cross, America’s Blood Centers, & the Armed Services Blood Program [AABB, ARC, ABC & ASBP], 2009).
Whole blood is stored at a temperature of 1°C (34°F) to 6°C (43°F) within 8 hours after collection. Expiration depends on the anticoagulant-preservative. The rate of administration for whole blood should be as rapid as necessary to correct and maintain the hemodynamic status. In massive hemorrhage, one or more large-gauge catheters, such as 14 or 16 gauge, allow a free-flow infusion of blood, although whole blood can be administered through either an 18- or a 20-gauge catheter. Electronic infusion devices do not deliver high enough rates to meet the needs of a clinical crisis. In this instance, pressure cuffs applied to the bag of blood may be required for rapid infusion. Further information is in the section on Blood Administration.
Irradiated Whole Blood and Blood Components
Irradiated whole blood and blood components have been exposed to a measured amount of radiation in order to prevent lymphocytes from replication. Irradiated blood components are used to prevent GVHD in patients with Hodgkin’s or non-Hodgkin’s lymphoma also
known as Hodgkin’s or non-Hodgkin’s lymphoma, acute leukemia, and congenital immunodeficiency disorders, patients with malignant tumors who are being treated with immunosuppressive therapy such as chemotherapy or radiation, low birth weight neonates, and patients treated with intrauterine infusions (
AABB, 2010). Irradiated units pose no risk to a transfusionist or the recipient. Because irradiation may damage and reduce the viability of RBCs, the original expiration date of the component prevails.
PLATELETS
Platelets are indicated for the control or prevention of bleeding in the presence of thrombocytopenia or abnormal platelet function. There are two preparations for platelets: random donor concentrates made from pooled units of platelets from several donors and concentrates obtained by platelet apheresis of a single donor. Units may be combined into a single bag before release from the blood bank. Platelets should not be infused through the same tubing as RBCs since some platelets are not ABO specific and may cause coagulation in the tubing. Platelets contain
factor III (tissue thromboplastin), a phospholipid that enhances the conversion of prothrombin to thrombin, which forms one of the most important steps in the coagulation process.
Table 17-3 describes coagulation factors.
Platelets, Pooled
Clinical conditions that adversely affect platelet function include sepsis, diseases of the liver and kidney, some bone marrow disorders, and congenital and acquired platelet disorders (
AABB et al., 2009). Platelet transfusions are indicated for thrombocytopenia caused by
hemorrhage with platelet counts of <50,000/µL, surgery with a platelet count of <50,000/µL, antineoplastic therapy with platelet counts of ≤10,000/µL, and in patients without bleeding whose counts are <15,000 to 20,000/µL and are rapidly decreasing. Major invasive procedures require platelet counts of at least 50,000/µL.
The decision to transfuse platelets must be based on the clinical condition of the patient, the cause of the thrombocytopenia, and the ability of the patient to produce platelets. Patients who are stable and are thrombocytopenic may tolerate platelet counts of <5,000/µL with minor (but not serious) bleeding. Platelets are not routinely indicated for patients with conditions in which rapid platelet destruction occurs such as in idiopathic thrombocytopenic purpura (ITP) or untreated disseminated intravascular coagulation (DIC), except in the presence of life-threatening bleeding (
AABB et al., 2009). For patients with thrombotic thrombocytopenic purpura (TTP) and heparin-induced thrombocytopenia with thrombosis, transfusion with platelets should be avoided due to the risk of fatal thrombosis, except in the setting of life-threatening hemorrhage.
Platelet concentrate prepared from whole blood contains a minimum of 5.5 × 10
10 platelets in 40 to 70 mL of plasma (
AABB et al., 2009). Each unit is expected to increase the platelet count of a 70-kg adult by 5,000 to 10,000/µL. The usual dose in an adult is 4 to 6 units and may be repeated in 1 to 3 days owing to the short life span of 3 to 4 days. Routinely, random donor platelets are pooled and transfused, and patients are the recipients of multiple allogeneic donors (
AABB, 2010).
Since ABO antigen is present on the platelet membrane, it is ideal to give ABO identical platelets. However, if a matching unit is not available and prompt transfusion is required, patients may receive any ABO group (
AABB, 2011). Platelet concentrates do not require cross-matching before infusion; however, documentation of the patient’s ABO and Rh status is necessary to make appropriate selection decisions. D-negative patients should receive compatible platelets, however, if available. Platelets may be infused as fast as the patient tolerates but must be infused within 4 hours after release from the blood bank (
AABB, 2010). Platelets may be stored at 20°C to 24°C for 5 days with gentle agitation. Platelets are never put into a refrigerator or cooler.
Platelets, Single Donor
Because of immune or nonimmune mechanisms, as many as 70% of patients who receive repeated platelet transfusions will become refractory to platelet transfusions and will not have an adequate sustained rise in their platelet counts after transfusion. This is due to the destruction of platelets by HLA antibodies soon after the transfusion is completed (
AABB, 2010). Causes of refractoriness to platelets also include DIC, drug therapies, ITP, and sepsis (
AABB, 2010). These patients are more likely to benefit by receiving HLA-compatible blood transfusions or receiving platelets that lack the antigens to which the recipient’s antibodies will not react.
Single-donor platelets are collected by platelet pheresis from a single donor who is HLA compatible with the recipient. Family is often a more suitable match; however, a nonrelated donor may be found. Pheresis platelets contain approximately ≥3 × 1011 per bag in about 250 mL of plasma. The platelet infusion volume may be between 100 and 500 mL supplied in a large plastic pack or in two connected packs to improve platelet viability by providing more surface area for gas exchange. The exact number of platelets in the unit can be obtained from the blood bank. Reactions to platelets may include disease transmission, GVHD, alloimmunization, febrile or allergic effects, and circulatory overload.
Platelets, Leukocytes Reduced
Leukocyte-reduced platelets are used to prevent recurrent febrile, nonhemolytic transfusion reactions that are caused when the donor white cell antigens and/or cytokines released during storage react to the recipient white cell antibodies (
AABB, 2010). Leukocyte removal may be accomplished by filtration resulting in the removal of 99.9% of the leukocytes at the bedside. The manufacturer’s guidelines for priming the filter must be adhered to for effective administration. Leukocyte reduction RBC filters and leukocyte reduction platelet filters are not interchangeable (
AABB, 2010). Again, acute hypotension may occur when leukocyte reduction is completed at the bedside, particularly in patients taking ACE inhibitor medication. Leukocyte-reduced platelets are also used to decrease the incidence of HLA alloimmunization in patients who may require long-term platelet replacement or transplantation in the future.
GRANULOCYTES
Granulocytes, administered infrequently, are most often collected from donors stimulated by corticosteroids (
AABB, 2011). Single-donor units are generally reserved for neonates due to the small number of granulocytes per unit. Previously prescribed for gram-negative sepsis, the emergence of new antibiotic regimens, new recombinant growth factors, and improved management of infections have greatly decreased the use of this component. Granulocytes are used in neutropenic patients with absolute neutrophil counts <500/µL. Granulocytes migrate toward, phagocytize, and kill fungi and bacteria. Patients receive granulocytes when they have infections not responsive to antibiotics and have a reasonable chance of recovery of bone marrow function or for neonatal sepsis. Cultures to identify the offending organism should be done. Granulocyte transfusion is not effective in localized infections or infections caused by agents other than bacteria. These transfusions only temporarily improve the patient’s condition. There is a renewed interest in granulocyte transfusions owing to improved cell doses that can be obtained from donors using sedimenting agents and granulocyte colony-stimulating factors (
AABB, 2011).
A unit of granulocytes contains variable amounts of lymphocytes, platelets, and RBCs suspended in 200 to 300 mL of anticoagulant and plasma. The number of granulocytes in each concentrate is usually >1 × 10
10. The granulocyte donor must be ABO and Rh compatible because a unit of granulocytes contains a significant amount of donor RBCs (
AABB et al., 2009).
Hydroxyethyl starch (HES) may be used as a red cell-sedimenting agent and will be present in the final component (
AABB et al., 2009). Granulocytes are administered through a standard blood filter over 2 to 4 hours for both adult and pediatric patients. Depth-type microaggregate filters and leukocyte reduction filters should not be used.
Patients undergoing granulocyte transfusions are acutely ill, and adverse effects are common. Fever, chills, allergic reactions, alloimmunization, and pulmonary reactions may occur and can be managed with diphenhydramine, nonaspirin antipyretics, steroids, and slow administration of the unit. The transfusion should not be stopped unless severe life-threatening symptoms occur. If the recipient is seronegative and severely immunosuppressed, donor CMV-seropositive granulocytes should not be given. Granulocytes are generally irradiated to prevent GVHD (
AABB et al., 2009).
Granulocytes are stored at 20°C to 24°C (68°F to 75°F) for no more than 24 hours without agitation (
AABB et al., 2009). Granulocytes should be administered as soon after collection as possible because of the deterioration of granulocyte function during short-term storage. Granulocytes are never put into a refrigerator or cooler.
 PATIENT SAFETY
PATIENT SAFETY PATIENT SAFETY
PATIENT SAFETY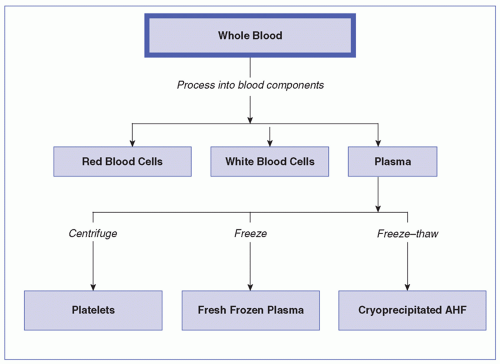
 EVIDENCE FOR PRACTICE
EVIDENCE FOR PRACTICE








