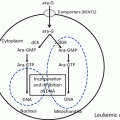
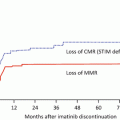
Fig. 8.1
Blinatumomab has anti-CD19 single-chain Fv (scFv) fragment at N-terminal and anti-CD3 scFv fragment at C-terminal. Each scFv is composed of V light chain and V heavy chain. Each scFv is connected by a flexible linker, which enables blinatumomab to bind a leukemic cell and a T cell
8.3 Mechanisms of Cytotoxicity of Blinatumomab
More than 95% of B-precursor ALL cells express CD19 on the leukemic cell surface [12]. Blinatumomab binds to both CD19 on the leukemic cell and to CD3 on T cells, thereby engaging T cells with CD19+ target leukemic cells and inducing cell toxicity. Blinatumomab can bind and activate both CD3 + CD4+ T cells and CD3 + CD8+ T cells. Because the activity of blinatumomab does not depend on the specificity of the T-cell receptor and does not require antigen presentation by major histocompatibility complex, leukemic cells are killed by polyclonal T cells [13]. Blinatumomab has two major effects on the T cell that is connected with the CD19+ leukemic cell through blinatumomab. The first effect is direct activation of the connected T cell, resulting in upregulation of the T-cell activation markers CD25, CD69, CD2, interferon (IFN)-γ, tumor necrosis factor (TNT)-α, interleukin (IL)-2, IL-6, and IL-10. The activated T cell then induces perforin-mediated cytotoxicity via granzyme entry into the CD19-positive leukemic cell. This leads to caspase activation and apoptosis of the CD19-positive leukemic cell. This effect is stronger in CD8-positive T cells than in CD4-positive T cells. The second effect is marked T-cell proliferation, leading to a supply of blinatumomab-activated T cells and serial killing of the CD19-positive target leukemic cells [14] (Fig. 8.2). This proliferation and serial killing of leukemic cells may be the reason why blinatumomab is effective for fully relapsed B-precursor ALL patients, who have so many target CD19-positive leukemic cells compared to the number of T cells. T-cell killing of CD19-positive leukemic cells and activated T-cell proliferation continue for as long as the T cell can make an immune synapse with a CD19-positive leukemic cell via blinatumomab, and they expire following elimination of the CD19-positive leukemic cell. By redirecting unstimulated human T cells to CD19-positive target cells, blinatumomab shows significant cytotoxicity at concentrations of 10–100 pg/ml [9]. This concentration is about 100 times lower than that of rituximab, which shows cytotoxicity at a concentration of 2 μg/ml [15]. In the presence of blinatumomab, the effecter-to-target ratio of the blinatumomab-activated T cell and the CD19-positive target cell is as low as 2:1, which is very low compared with other immunotherapies [9].
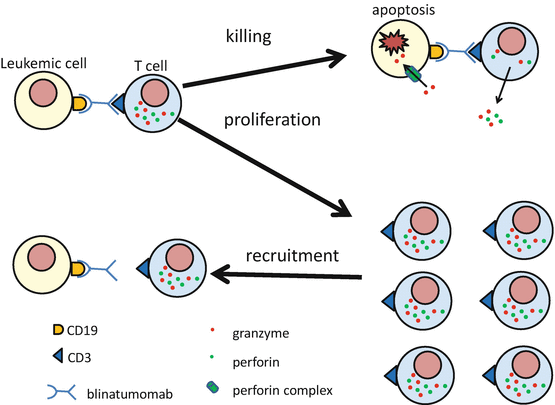

Fig. 8.2
Blinatumomab has two major effects to T cell, which is connected with CD19+ leukemic cell by blinatumomab. First effect is direct T-cell activation which results in killing of CD19+ leukemic cell with perforin-mediated cell cytotoxicity via granzyme entry. Second effect is T-cell proliferation. The proliferated T cells are recruited for serial killing of the CD19-positive target leukemic cell. Blinatumomab can bind to and activate both CD3 + CD4+ T cells and CD3 + CD8+ T cells. The activity of blinatumomab does not depend on the specificity of the T-cell receptor and not require antigen presentation by major histocompatibility complex
8.4 Pharmacokinetics of Blinatumomab
The serum half-life (t1/2) of blinatumomab is very short compared with that of a traditional monoclonal antibody such as rituximab, because blinatumomab lacks the Fc region of an antibody. The average t1/2 of blinatumomab and rituximab is 1.25 h and 76.3 h, respectively [14, 16]. This short t1/2 is the reason why continuous intravenous (CIV) administration of blinatumomab is needed, whereas traditional monoclonal antibodies are administered intermittently. The systemic clearance is not affected by creatinine clearance, age, gender, weight, or body surface area [14]. A phase 1 trial of blinatumomab was carried out for 38 patients with relapsed/refractory non-Hodgkin lymphoma. In this trial, blinatumomab was given as CIV administration, using doses ranging from 5 to 60 μg/m2 over a period of 4–6 weeks. This trial indicated that the maximum tolerated dose was 60 μg/m2 and that the majority of tumor responses were seen during the first 4 weeks [17]. In the cases of patients who received 15 μg/m2 of blinatumomab given as CIV administration, its serum concentration reached steady state within a day, and the steady-state concentration was 731 ± 163 pg/ml (mean ± SD; range: 492–1050 pg/ml) [14]. In vitro analysis, a cytolytic effect of blinatumomab was observed at concentrations between 10 and 100 pg/ml [9]. In this phase 1 study, tumor regression was observed in patients who were treated with 5 μg/m2 of blinatumomab given as CIV administration [17]. Based on these results, it was recommended that blinatumomab should be given by CIV administration at a dose greater than 15 μg/m2 for 4 weeks, with a 2-week rest between cycles.
8.5 Immunological Responses of Blinatumomab Infusion
8.5.1 Peripheral B-Cell Kinetics Induced by Blinatumomab Infusion
The number of B cells in peripheral blood that expressed CD19 on their surface rapidly decreased to a very low level with CIV administration of blinatumomab. The mean time to reach nadir was 2.18 ± 3.80 days (mean ± SD; range 0.003–13.94 days). The number of B cells in peripheral blood remained consistently very low during the treatment with blinatumomab, including the 2-week rest periods between cycles [14].
8.5.2 Peripheral T-Cell Kinetics Induced by Blinatumomab Infusion
In the setting of CIV administration of blinatumomab for MRD-positive ALL patients in CR, the number of peripheral T cell began to decrease within 1 h in response to the start of blinatumomab infusion. The nadir was reached within 0.36 ± 0.24 days (mean±SD; range 0.06–1.09 days), and the number of peripheral T cells at nadir was as low as 10% of that at baseline. After the nadir had been reached, the number of peripheral T cells gradually began to increase and had recovered to 50% of the baseline number after 3.13 ± 1.85 days (mean ± SD; range 0.82–9.10 days). The number of peripheral T cells had recovered to baseline on days 8–9 and continued to increase up to day 17, when the number of expanded peripheral T cells had increased to double the number that was present before the start of blinatumomab. Although the number of peripheral T cell decreased after 17 days, their number was maintained above the baseline during CIV administration of blinatumomab [14].
8.5.3 Profile of Expanded T Cells Induced by Blinatumomab Infusion
Analysis of the expanded peripheral T-cell subsets showed three types of patterns: greater expansion of CD4+ T cells than CD8+ T cells, greater expansion of CD8+ T cells than CD4+ T cells, and approximately similar expansion of CD4+ and CD8+ T cells. Except for a very few cases, expansion of regulatory T cells in peripheral blood was not induced by administration of blinatumomab. The majority of expanded CD8+ T cells in peripheral blood were effecter memory T cells of CD45RA−/CD197 phenotype [14].
8.5.4 Cytokine Profiles Induced by Blinatumomab Infusion
Engagement of T cells with leukemic cells through blinatumomab activates T cells, and activated T cells produce inflammatory cytokines including IL-2, IL-6, IL-10, IFN-γ, and TNF-α. The levels of these cytokines in peripheral blood reached a maximum within 1 day after the start of blinatumomab administration and dropped to undetectable levels within 3 days after the start of blinatumomab administration. The elevation of inflammatory cytokines is not observed in patients with a low tumor burden because T-cell activation occurs when an immune synapse is made between a T cell and a CD19+ leukemic cell via blinatumomab [14].
8.6 Safety and Adverse Effects
8.6.1 Adverse CNS Events
The most common adverse CNS events are tremor (17%), dizziness (14%), confusion (7%), encephalopathy (5%), ataxia (5%), and somnolence. In many cases, these adverse CNS events are reversible with temporal interruption of blinatumomab, dexamethasone, and anticonvulsant, but they force the discontinuation of administration of blinatumomab. For the patients whose neurological adverse events are less than grade 4, blinatumomab can be reintroduced starting at the lowest dose (5 μg/m2) after these events have resolved [18]. CNS events tend to occur in patients with CNS infiltration of leukemic cells or in those with underlying CNS diseases. These data suggest that blinatumomab should not be given to such patients. The mechanism of blinatumomab-induced CNS toxicity could involve direct T-cell-mediated toxicity and/or cytokine-mediated toxicity such as the CNS toxicity that is induced by CAR T-cell therapy [19]. These are the reasons why adverse CNS events tend to occur in patients who have a high tumor burden or in those who were given blinatumomab for the first time.
8.6.2 Cytokine Releasing Syndromes
Cytokine releasing syndromes (CRS) develop as the result of systemic inflammation that is caused by inflammatory cytokines such as IL-2, IL-6, IL-10, IFN-γ, and TNF-α, which are produced by blinatumomab-activated T cells. In most CRS cases, symptoms are flu-like such as pyrexia and myalgia. In severe CRS cases, inflammatory cytokines induce capillary leak and result in hypotension, pulmonary edema, coagulopathy, and multi-organ failure. The preventive administration of dexamethasone (10 mg/m2/day) during the first 5 days of blinatumomab infusion and dose reduction of blinatumomab to 5 μg/m2/day (or 9 μg/day) during the first 7 days of blinatumomab effectively prevent development of CRS and do not affect the result of blinatumomab treatment. CRS usually develops in the first cycle of blinatumomab treatment, because T-cell activation and inflammatory cytokine release occur due to immune synapse formation between T cells and CD19-positive leukemic cells via blinatumomab [18, 20].
8.6.3 Hypogammaglobulinemia
Because B cells differentiate into plasma cells that produce immunoglobulins, B-cell elimination by blinatumomab results in hypogammaglobulinemia. Since CD19, but not CD20, expression is maintained on plasma blast cells, the period of hypogammaglobulinemia resulting from blinatumomab administration is prolonged compared to that resulting from an anti-CD20 antibody such as rituximab. Although the levels of all immunoglobulin classes in serum are decreased by treatment with blinatumomab, the greatest decrease is seen in the level of serum IgA. The serum levels of IgG, IgM, and IgA fall to 29%, 12%, and 6% of their respective baseline values. The level of each immunoglobulin class begins to recover in the order of IgM, IgG, and IgA after completion of blinatumomab treatment. It takes a long time to recover the level of serum immunoglobulins after completion of blinatumomab treatment because plasma cells have to be differentiated from naïve and memory B cells, which are regenerated by CD19-negative B-cell progenitors. Within 2 years, the levels of serum IgG and IgM, but not the levels of serum IgA, recover to more than 50% of the level of the pretreatment baseline. Because immunoglobulin is an important humoral factor for the eradication of foreign pathogens, it might be necessary to regularly monitor the level of serum immunoglobulin and to replenish it to the minimum level to prevent infection [21].
8.6.4 Other Adverse Effects
Cytopenias are frequently observed during blinatumomab treatment. It is difficult to determine whether these cytopenias are related to the effects of blinatumomab or to ALL itself. Neutropenia and febrile neutropenia (FN) more than grade 3 were developed in 25% and 16%, respectively, of ALL patients treated with blinatumomab [18]. It is necessary to pay attention to infectious disease both during and after treatment with blinatumomab, because hypogammaglobulinemia induced by blinatumomab results in patients with a compromised immune status.
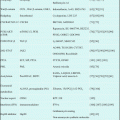
8.7 Results of Clinical Trials of Blinatumomab (Table 8.1)
8.7.1 Clinical Results of Adult Patients with Relapsed or Refractory B-Precursor ALL Treated with Blinatumomab
Two results of phase 2 clinical trials of blinatumomab have been published to date. The first report included 36 relapsed or refractory B-precursor ALL patients with or without Philadelphia chromosome, including 21 patients who had received allo-HSCT before the treatment with blinatumomab. The median age was 32 years (range: 18–77 years of age). At the beginning of this trial, blinatumomab was administered at a dose of 15 μg/m2 as CIV administration for 4 weeks with a 2-week rest between cycles. Because grade 4 CRS was observed, prophylactic treatment with dexamethasone at a dose of up to 24 mg for up to 5 days and/or 200 mg/m2 of cyclophosphamide for up to 4 days was permitted, and the initial dose of blinatumomab in the first week of treatment was lowered to 5 μg/m2 and was later increased to 15 μg/m2. Sixty-nine percent of patients achieved a CR or CR with partial hematologic recovery (CRh), with 88% of responders achieving an MRD response. Forty-two percent of patients who achieved CR or CRh underwent subsequent allo-HSCT. The median overall survival (OS) and relapse-free survival (RFS) were 9.8 and 7.6 months, respectively. Frequent adverse events (AEs) during the treatment were pyrexia (81%), fatigue (50%), headache (47%), tremor (36%), and leukopenia (19%). The most frequent AEs of grade 3 or 4 were transient leukopenia and thrombocytopenia. Infectious diseases developed in 12 patients, including 5 deaths. None of the patients that died from infectious disease had achieved a CR or CRh. CNS events were observed in 22% of the patients. Two patients developed grade 4 CRS, and none of the nonresponders to blinatumomab treatment developed CRS [20].
Table 8.1




Results of clinical trials of blinatumomab
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree