VWF:Ag
VWF:RCo
VWF:RCo/VWF:Ag
FVIIII
Multimer pattern
LD-RIPA
Platelet count
Other specialized testing
Type 1
Mild decrease
Mild decrease
>0.5–0.7
Normal or mild decrease
Normal
Absent
Normal
Type 1C
Mild decrease
Mild decrease
>0.5–0.7
Normal or mild decrease
Normal
Absent
Normal
Increased VWFpp/VWF:Ag
Type 2A
Absent
Moderate decrease
<0.5–0.7
Normal or mild decrease
Loss of HMW multimers
Absent
Normal
Abnormal VWF:collagen binding assay
Type 2B
Mild decrease
Moderate decrease
<0.5–0.7
Normal or mild decrease
Loss of HMW multimers
Increased
Decreased
Abnormal VWF:platelet binding assay
Type 2M
Mild decrease
Moderate decrease
<0.5–0.7
Normal or mild decrease
Normal
Normal
Normal
Type 2N
Normal or mild decrease
Normal or mild decrease
<0.5–0.7
Moderate decrease
Normal
Normal
Normal
Abnormal VWF:FVIII binding assay
Type 3
Absent
Absent
>0.5–0.7
Absent
Absent
Normal
Platelet type
Mild decrease
Moderate decrease
<0.5–0.7
Normal or mild decrease
Loss of HMW multimers
Decreased
Normal VWF:platelet binding assay
Genetics
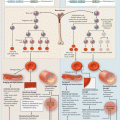
The VWF gene is located near the short arm of chromosome 12. It spans 178 kb and contains 52 exons [6]. VWF mutations that cause VWD include large deletions, frameshift mutations, nonsense mutations, and missense mutations. Mature VWF consists of 3 A and B domains, 2 C domains, and 4 D domains. The specific domain location of the VWF gene mutation often correlates with a particular VWD variant [7]. To date, 70 % of those with type 1 VWD, and nearly all of those with type 2 and 3 VWD, have detectable genetic abnormalities that cause the disease. Type 1 VWD usually involves single amino acid substitutions in the D3 domain. Type 2A VWD involves mutations in the D3 or A2 domains. Types 2B, 2M, and 2N VWD include mutations in the A1, A3, and D3 domains, respectively. Types 3 VWD usually involves nonsense and frameshift mutations and occurs throughout the VWF gene [3]. All VWD variants are inherited in an autosomal dominant fashion except for type 2N VWD and type 3 VWD, which are inherited autosomal recessively.
Clinical Manifestations
VWD is a disorder of primary hemostasis; therefore, bleeding manifestations are primarily mucocutaneous in origin. The most common bleeding symptoms include epistaxis, easy bruising, menorrhagia, gingival bleeding, gastrointestinal bleeding, and postoperative bleeding. VWD is often overlooked as a cause of menorrhagia; however, it is a common presenting symptom in women with VWD and should be considered in the appropriate clinical context. Angiodysplasia is uncommon, but usually associated with type 2 or 3 VWD, and should be considered in any VWD patient with chronic gastrointestinal bleeding [8]. The occurrence of hemarthroses and hematomas is uncommon except in the more severe forms of the disease, such as type 3 VWD. Bleeding commonly occurs following hemostatic challenge, such as surgery, dental extractions, menarche, and childbirth. With the latter, it is critical to recognize that while bleeding occurs early, it may persist as long as 6 weeks postpartum. Clinical bleeding symptoms are often variable within families, and even within the same person at different times.
Diagnostic Studies
Laboratory testing for the diagnosis and classification of VWD is imperfect (Table 6.1) [2]. VWF and FVIII are acute phase reactants; therefore, levels are increased with inflammation, trauma, and stress. Several other factors affect VWF levels, including estrogen, pregnancy, and blood type. In the appropriate clinical scenario, normal testing does not necessarily exclude the presence of VWD and may need to be repeated on multiple occasions.
Assays to measure VWF antigen (VWF:Ag), VWF ristocetin cofactor (VWF:RCo), and FVIII levels are the initial tests performed when VWD is suspected. These tests are commonly available at most hospitals. VWF:Ag is an immunoassay used to the measure the concentration of VWF in plasma. Most laboratories used enzyme-linked immunosorbent assay (ELISA) or automated latex immunoassay (LIA). VWF:RCo is an assay performed to measure the functional activity of VWF based on ristocetin-induced platelet agglutination (RIPA). Ristocetin is an antibiotic that triggers VWF-platelet binding. Despite significant intra- and inter-laboratory variation, VWF:RCo is still the most sensitive and specific test available for measuring VWF function [9]. FVIII levels are measured in a functional assay based on the activated partial thromboplastin time (aPTT) to determine coagulant activity. For all of the above tests, reference ranges vary by laboratory but the normal range is generally considered to be 0.50 IU/dL to 2.00 IU/dL.
Depending on the results of the above tests, further testing may be indicated. While VWF:Ag and VWF:RCo levels less than 0.30 IU/dL are indicative of VWD based on the higher presence of genetic mutations in patients with these levels, generally VWF:Ag and VWF:RCo levels less than 0.50 IU/dL are considered clinically diagnostic of VWD. Decreased VWF:Ag levels and normal or comparably decreased VWF:RCo levels are consistent with type 1 VWD. Undetectable or nearly undetectable VWF:Ag and VWF:RCo levels along with significantly reduced FVIII levels are consistent with type 3 VWD. Type 2 VWD is suspected when VWF:RCo levels are decreased out of proportion to VWF:Ag levels. A ratio less than 0.5 to 0.7 can help distinguish type 2 VWD from type 1 VWD [10]. If type 2 VWD is suspected, multimer analysis is helpful in determining the specific subtype of type 2 VWD. Multimer analysis is done using agarose gel electrophoresis to separate out VWF proteins (multimers) by molecular weight followed by visualization with immunostaining. Loss of high molecular weight multimers is consistent with type 2A or 2B VWD. Type 2M and 2N have normal multimer analyses. Type 1 VWD has a reduced staining intensity, which affects all markers. In type 3 VWD, all markers are absent or barely visible. Differentiating between type 2A and 2B VWD can be done using the platelet count, which is often decreased in type 2B VWD, and RIPA. Ristocetin does cause VWD-platelet binding at low concentrations, less than 0.6 mg/mL; however, VWF hyperresponsiveness to ristocetin at these low concentrations in type 2B VWD results in increased VWF-platelet binding and platelet agglutination at rest. Other less commonly used tests, include the VWF: platelet binding assay, which will help distinguish type 2B VWD from platelet-type VWD; the VWF:collagen binding assay to assist in differentiating type 2A VWD from type 2M VWD; and the VWF:factor VIII binding assay to aid in diagnosing type 2N VWD.
Treatment
Therapy for VWD is generally aimed at increasing VWF levels in type 1 VWD and replacing abnormal VWF in types 2 and 3 VWD. In type 1 VWD, VWF can be increased by stimulating the release of endogenous VWF from endothelial stores with DDAVP (1-desamino-8-D-arginine vasopressin) or by providing VWF replacement with the use of VWF-containing plasma concentrates . DDAVP is a synthetic analogue of vasopressin. It can be administered intranasally or intravenously. Nasal administration of DDAVP in the form of Stimate is indicated for minor bleeding, such as epistaxis, or minor wounds. One spray of 150 μg in one nostril is administered to patients weighing less than 50 kg, and one spray of 150 μg in each nostril (totaling 300 μg) is given for those weighing 50 kg or more. Intravenous DDAVP is administration in 50 mL of normal saline over 30 min at a dose of 0.3 μg/kg. This is reserved for more significant minor bleeding or minor surgical procedures, such as uncomplicated dental extractions. The maximum response to DDAVP is seen 30–90 min following administration. DDAVP is generally given every 24 h for a maximum of three doses before clinical responsiveness disappears due to exhaustion of endogenous VWF stores. Potential adverse effects of DDAVP include flushing, headache, nausea, allergic reaction, thrombosis, and water retention. In individuals receiving more than 1.5 L of fluids daily, such as in the postoperative setting, and more commonly in children, water retention can result in hyponatremia and seizures; therefore, it is very important to limit fluid intake to 1.5 L for 24 h after receiving DDAVP [11]. DDAVP is usually indicated for mild type 1 VWD. It is less commonly effective in type 2 VWD and is ineffective in type 3 VWD. Approximately 20 % of type 1 VWD and most type 2 and 3 VWD patients do not respond to DDAVP; therefore, it is recommended that all patients with type 1 VWD undergo a “DDAVP challenge” to assess response to DDAVP, in terms of VWF:Ag, VWF:RCo, and FVIII levels [12]. This should be done at least 3 weeks before anticipation of using DDAVP for a surgery. If the DDVAP challenge is undertaken in less than 3 weeks, VWF stores will not be able to replenished (tachyphylaxis), and response will be limited when it is needed.
Plasma-derived, virus-inactivated VWF-containing concentrates are indicated for VWD in patients unresponsive to DDAVP as well as significant bleeding or major surgical procedures, which will require treatment for longer than 3 days (due to tachyphylaxis). The most commonly used VWF-containing concentrates are Humate-P and Alphanate (Antihemophilic factor/von Willebrand complex [human]). The dosing and duration of therapy is largely empiric and based on expert opinion. Usual practice for surgery involves administering VWF concentrate 80 units/kg by IV slow push, followed by 50 units/kg every 8–12 h for 3–5 days thereafter. Potential adverse effects are uncommon but may include allergic reaction and thrombosis. Recently, the first plasma-free recombinant version of VWF has been studied in clinical trials with promising results and is currently awaiting FDA approval [13].
Other potential therapy includes antifibrinolytic agents , ε-aminocaproic acid and tranexamic acid, which are usually given as adjunctive therapy to DDAVP or VWF concentrates for effective control of mucosal bleeding, especially for menorrhagia or dental surgery. Their use may be limited by the potential to cause nausea.
Hemophilia
Background
Hemophilia is an X-linked hereditary bleeding disorder characterized by deficient or defective coagulation factors VIII (FVIII), in hemophilia A, and IX (FIX), in hemophilia B. Hemophilia A is more common than hemophilia and accounts for 80–85 % of the hemophilia population. Hemophilia A occurs in 1 in every 5000–7000 live male births, and hemophilia B occurs in 1 in every 25,000 to 30,000 live male births [14]. There are approximately 400,000 individuals worldwide affected by hemophilia [15]. Both diseases occur in all races and ethnicities and have no geographic predilection.
FVIII acts as a cofactor for FIX in the coagulation cascade. Following activation by thrombin, activated FVIII (FVIIIa) associates with activated FIX (FIXa) forming the “tensase” complex with factor X (FX). FVIIIa functions to increase the rate of FX activation by FIXa [16]. FX ultimately activates prothrombin, generating thrombin and promoting clot formation. Both FVIII and FIX deficiency leads to reduced thrombin generation and weak and unstable clots.
Genetics
Hemophilia is a classic example of an X-linked recessive disorder . FVIII and FIX genes are located on the long arm of the X chromosome. All female offspring of affected males will inherit the defective X chromosome, and all male offspring will be unaffected. Females inheriting one affected X chromosome are obligate carriers and generally do not develop bleeding manifestations except in the presence of lionization, which results in a greater percentage of the normal FVIII allele being inactivated. 50 % of male offspring of female carriers will have hemophilia, and 50 % of female offspring will be carriers themselves. Approximately, one-third of hemophilia cases result from a spontaneous mutation and no known family history of hemophilia or abnormal bleeding can be identified [17].
The FVIII gene spans 186KB and contains 26 exons [18]. The FIX gene is much smaller, comprised of 33KB with 8 exons [19]. More than 1000 disease-causing mutations have been identified in each gene [20, 21]. The type of mutation often correlates with the severity of disease. Large deletions are associated with severe hemophilia. Alternatively, point mutations are more likely to result in moderate or mild hemophilia. One of the most commonly identified FVIII gene mutations is the intron 22 inversion. Mutations involving CpG dinucleotides account for a significant number of disease-causing mutations involving the FIX gene [22].
Clinical Manifestations
Hemophilia is categorized into three levels of severity: mild (baseline factor level 5–40 %), moderate (baseline factor level 1–5 %), and severe (baseline factor level <1 %). Individuals with mild or moderate hemophilia usually experience bleeding following provocation only, such as trauma or surgery. Severe hemophilia is associated with spontaneous bleeding.
Hemarthroses
The most common bleeding manifestation experienced in hemophilia is joint bleeding, referred to as hemarthrosis, which accounts for 75 % of bleeds [23]. Bleeding most commonly involves large, weight-bearing joints, such as the knees. Other commonly affected joints are the ankles and elbows. Intraarticular bleeding originates from blood vessels in the synovial lining of the joint. Bleeding results in an inflammatory response, and patients initially experience a tingling sensation, referred to as an aura, followed by the development of pain. Physical examination most often reveals a warm and swollen joint with reduced range of motion. Left untreated, pain will progressively worsen. Complications include the potential for recurrent spontaneous bleeding in the affected joint over a short period of time, referred to as a target joint. Other complications of hemarthroses include the potential for chronic hemophilic arthropathy (CHA) . Residual intraarticular blood results in chronic inflammation, synovitis, and synovial hypertrophy. This predisposes to further bleeding, loss of articular cartilage, and progressive joint destruction. Along with damage of adjacent bony structures, CHA manifests with chronic joint pain, loss of motion, muscle atrophy, and contractures.
Soft-Tissue Hematomas
Another common manifestation of hemophilia is bleeding into subcutaneous tissues and muscle. The most commonly affected areas are the calves, thighs, and forearms although spontaneous bleeding may occur at any location. If not promptly treated, hemorrhage may have devastating consequences, such as neurovascular compromise and compartment syndrome in extremity bleeds, and life-threatening airway compromise in pharyngeal hematomas, among others. Iliopsoas bleeding is possible and manifests as pain in the groin, back, and/or abdomen. Recurrent hemorrhages may lead to muscle atrophy and contractures.
Other Bleeding Manifestations
While hemarthroses and soft tissue hematomas are the most commonly encountered manifestations of bleeding in hemophilia, spontaneous bleeding may occur at any location. Life-threatening bleeding includes central nervous system hemorrhage, gastrointestinal bleeding, retroperitoneal hematoma, and hemorrhage involving the neck and/or pharynx. Other non-life threatening bleeding includes oral mucosal bleeding, epistaxis, and hematuria.
Diagnosis
The aPTT is prolonged in hemophilia except in mild cases where factor levels are greater than 20–25 %. A mixing study is performed in the initial evaluation of an isolated prolonged aPTT. Correction of the prolonged aPTT is indicative of an underlying factor deficiency, whereas failure of the prolonged aPTT to correct is worrisome for the presence of a factor inhibitor. Once a factor deficiency is suspected, specific factor assays can identify the deficient factor. A one-stage clotting assay will reveal decreased FVIII and FIX levels in hemophilia A and B, respectively.
Treatment
Recombinant factor FVIII (rFVIII) and IX (rFIX) are the preferred treatments for hemophilia A and B, respectively, in the absence of an inhibitor (see Complications below). Current rFVIII products include Recombinate, Kogenate FS, Helixate FS, Advate, and Xyntha, and rFIX products include Benefix and Rixubis. Recently, the FDA approved the use of rFVIII Fc fusion protein (Eloctate) and rFIX Fc fusion protein (Alprolix) for the treatment of hemophilia A and B, respectively. The Fc portion of IgG1 binds to the neonatal Fc receptor, delaying lysosomal degradation, and extending the half-life of rFVIII from 8 to 12 to 20 h and rFIX from 12 to 20 to 87 h [24, 25]. Other extended half-life factor products, including pegylated and albumin-fusion proteins, which are in various stages of clinical trials are expected to be available for clinical use in the near future.
All bleeds should be treated as soon as recognized to limit morbidity and chronic inflammation. All patients should have factor stored at home for home infusion as needed. Patients with hemophilia should refrain from all aspirin, antiplatelet, and nonsteroidal antiinflammatory containing drugs. Medications known to cause platelet dysfunction should be avoided. There is no strong evidence supporting treatment guidelines for acute bleeding and surgical prophylaxis in hemophilia, and standard practice is based on consensus expert opinion and varies among different hemophilia treatment centers. An overview of our hemophilia center’s practice is presented in Table 6.2. As the optimal treatment with extended half-life products in the setting of surgery has not yet been established, and, as the cost would increase with more frequent use in the hospital setting, we reserve use of extended half-life products for prophylaxis in the outpatient setting, while continuing to recommend standard rFVIII and rFIX for surgery and use in the hospital. In an adult, 1 unit of FVIII per kg of body weight is expected to raise the FVIII level 2 %, and 1 unit of FIX per kg of body weight is expected to raise the FIX level 1 %; therefore, 50 and 100 units/kg of FVIII and FIX, respectively, are necessary to raise the factor level to 100 % [26]. The availability of safe and effective factor therapy dramatically changed the outlook for a generation of patients with hemophilia. The use of factor therapy as prophylaxis prior to the onset of joint disease has allowed many children with hemophilia to reach adulthood with minimal or no significant joint disease [27]. The goal of prophylaxis is to maintain factor levels above 1 %, which greatly diminishes the risk of spontaneous bleeding. Prophylaxis regimens used at our hemophilia center are detailed in Table 6.2. Adjunctive therapies include DDAVP for patients with mild hemophilia A, but it should only be used after an adequate response to treatment has been demonstrated. Antifibrinolytic therapy is effective for oral mucosa bleeding, but its use may be limited by nausea.
Table 6.2
Hemophilia management strategiesa
Hemophilia A | Hemophilia B | |
|---|---|---|
Acute bleed | ||
Minor (joint or soft tissue) | 50 units/kg initially then 25 units/kg daily × 1–3 days | 100 units/kg initially then 50 units/kg daily × 1–3 days |
Major (abdominal, gastrointestinal, thoracic) | 50 units/kg initially then 25 units/kg every 8 h × 3 doses then 25 units/kg every 12 h × 2 doses then 25 units/kg daily × 2 weeks | 100 units/kg initially then 50 units/kg every 12 h × 2 doses then 50 units/kg daily × 2 weeks |
CNS | 50 units/kg initially then 25 units/kg every 8 h × 3 doses then 25 units/kg every 12 h × 2 doses then 25 units/kg daily × 3 weeks | 100 units/kg initially then 50 units/kg every 12 h × 2 doses then 50 units/kg daily × 3 weeks |
Bleeding prophylaxis | 25 units/kg every Monday and Wednesday and 50 units/kg every Friday | 50 units/kg every Monday and 100 units/kg every Thursday |
Surgical prophylaxis | ||
Minor (biopsy, laparoscopic, arthroscopic) | 50 units/kg immediately prior to surgery then 25 units/kg 8–12 h postoperatively then 25 units/kg daily × 1–5 days | 100 units/kg immediately prior to surgery then 50 units/kg 12 h postoperatively then 50 units/kg daily × 1–5 days |
Major (cardiac, CNS, orthopedic) | 50 units/kg immediately prior to surgery then 25 units/kg every 8 h × 2 days then 25 units/kg every 12 h × 2 weeks then 25 units/kg daily × 1 week | 100 units/kg immediately prior to surgery then 50 units/kg every 12 h × 2 weeks then 50 units/kg daily × 1 week |
Dental | 50 units/kg immediately prior to surgery then 25 units/kg 8–12 h postoperatively then 25 units/kg daily × 10 days (alternatively aminocaproic acid can be substituted) | 100 units/kg immediately prior to surgery then 50 units/kg 12 h postoperatively then 50 units/kg daily × 10 days (alternatively aminocaproic acid can be substituted) |
Complications
Chronic Hemophilic Arthropathy
Chronic hemophilic arthropathy is best treated through prevention with the regular use of factor infusions. Once present, chronic pain is best managed with acetaminophen or the judicious use of opioids in consultation with a pain medicine specialist. Physical therapy and orthotics can improve function of diseased joints significantly. Ultimately, orthopedic surgery, such as joint replacement or fusion, may be necessary if pain and functionality are refractory to more conservative management and having a significantly negative impact on quality of life.
Viral Infections
In the 1980s, a large percentage of patients with hemophilia receiving treatment with plasma-derived factor concentrates were infected with chronic hepatitis C virus (HCV) and human immunodeficiency virus (HIV). This was devastating to the hemophilia population resulting in significant morbidity and mortality over the course of the next several decades. However, HIV can now be managed as a chronic disease with the use of highly active antiretroviral therapy. Furthermore, chronic HCV is now curative in most patients receiving direct acting antiviral agents, such as sofosbuvir and ledipasvir (Harvoni). Most importantly, the risk of transmission of blood-borne viruses has been virtually eliminated in hemophilia patients with the use of recombinant factor products and a much safer blood supply.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree