Author (year)
n
RT course
RT dose (Gy)
OS
CSS
CR (%)
Salvage RC
(%)
aHousset et al. (1993)
54
Split course
44 (bid)
3y, 59%
3y, 62%
74
–
aShipley et al. (1998)
62
Split course
64.8
5y, 49%
–
60
25.8
aJames et al. (2012)
182
Continuous
55–64
5y, 48%
–
–
11.4
aTunio et al. (2012)
200
Continuous
65
5y, 52%
–
93
–
Kaufman et al. (2000)
34
Split course
44 (bid)
–
3y, 83%
67
29.4
Hussain et al. (2004)
41
Continuous
55 Gy in 20
2y, 50%
2y, 68%
71
19.5
Peyromaure et al. (2004)
43
Split course
24 Gy in 8 (bid)
–
3y, 75%
74.4
25.6
Kragelj et al. (2005)
84
Continuous
64
9y, 25%
9y, 51%
78
8.3
Cogna et al. (2006)
113
Continuous
64
–
5y, 50%
70
15
Weiss et al. (2007)
112
Continuous
55.8–59.4
5y, 74%
5y, 82%
88
17
Aboziada et al. (2009)
50
Split course
66
1.5y, 100%
1.5y, 84%
60
28
Choudhury et al. (2011)
50
Continuous
52.5 Gy in 20
5y, 65%
5y, 78%
82
14
Lagrange et al. (2011)
51
Split course
63
8y, 36%
–
–
33.3
Zapatero et al. (2011)
39
Split course
64.8
5y, 73%
5y, 82%
80
33
At the University of Erlangen, the authors retrospectively analyzed 415 patients with MIBC. The treatment included TUR-BT followed 4 weeks later by RCT up to a dose of 45–54 Gy to the bladder and pelvic nodes, and then whole bladder dose is boosted to 55.8–59.4 Gy total dose, depending upon the completeness of TUR-BT. Six to eight weeks after completion of therapy, response is assessed by cystoscopy. If the response is incomplete, cystectomy is indicated. The complete response rates for all patients and radiotherapy alone were 72% and 61%, respectively. The 5- and 10-year OS rates were 51% and 31%, while salvage cystectomy rates were 20% [40].
The Bladder Cancer 2001 (BC2001) study was the first randomized study investigating the role of chemotherapy to TUR-BT followed by radiotherapy in MIBC [31]. The chemotherapy regimen consisted of 5-FU and MMC. The 2-year DFS was significantly improved in the combined treatment arm compared to RT alone (67% vs. 54%, p = 0.03). Also there was a trend favoring RCT arm in 5-year OS rates (48% vs. 35%; p = 0.16). The National Cancer Institute of Canada (NCIC) investigated the addition of cisplatin chemotherapy to RT for organ-sparing treatment modality [25]. There was a statistically significant reduction in pelvic recurrences in patients treated with concurrent RCT compared to RT alone (29% vs. 52%); however, no statistically significant difference in OS was observed.
Several investigative protocols were carried out by the “Radiation Therapy Oncology Group” (RTOG). The first one, RTOG 85-12 , consisted of induction RT with cisplatin; thereafter patients with complete response received additional RT and a third dose of cisplatin [41]. In RTOG 88-02 study, the toxicity of adding neoadjuvant, cisplatin, methotrexate, and vinblastine (CMV) to the combined treatment was evaluated [42]. The good tolerability of the regimen in this study led to RTOG 89-03, a randomized phase III trial assessing the efficacy of neoadjuvant CMV [27]. However, this study was stopped early due to unacceptably high toxicity rates in the CMV arm. Moreover, the addition of neoadjuvant CMV did not show any benefit in terms of complete response and overall survival or bladder preservation rates. RTOG 95-06 evaluated the accelerated hypofractionated scheme [30]. Although overall survival and bladder preservation rates were encouraging, grade 3 or more genitourinary toxicity rates were a concern. RTOG 97-06 also evaluated the efficacy of hypofractionated RT scheme [43]. In RTOG 99-06 , paclitaxel was added to the concomitant cisplatin and gemcitabine in the adjuvant setting leading to an excellent complete rate of 87% [44].
Radiation dose and schedule vary within countries, but the most acceptable fractionation is 40–46 Gy to the pelvic lymph nodes and bladder with a boost to tumor a total dose of 60–66 Gy. Cisplatin is the most common concurrent chemotherapy agent. The main goal of this chemotherapy is being a radiosensitizer. After performing TUR-BT, the bladder preservation protocols usually belong to one of the three categories (Fig. 4.1). In the first scheme RT is delivered to patients who attain pathological complete remission after a full dose of chemotherapy. The second scheme includes induction chemotherapy with two to three cycles of CMV and then RCT for the responders. The third scheme entails concomitant RCT either with moderate dose followed by cystoscopy and consolidation RCT or to give high-dose RCT and to perform cystoscopy after completion of the dose. For all schemes, cystectomy is indicated for those who attained non-complete response. A cystoscopy with re-biopsy should be performed for assessment of therapy response. This evaluation could be done at the end of TMT (continuous course) or before planning to the boost volumes after 40–46 Gy (split course). In the latter, patients responding to the treatment were treated for boost to the tumor, while nonresponders were offered for salvage RC. Five-year overall survival (OS) and cancer-specific survival rates range from 36% to 74% and 50% to 82%, respectively. Salvage cystectomy rates were 25–30% [45].
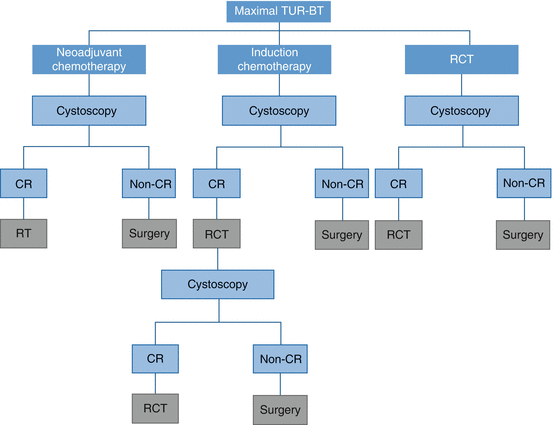

Fig. 4.1
Bladder preservation protocols usually belong to one of the three categories
Advanced age is not a contraindication for TMT. As older patients are more likely to have significant comorbidities, treatment should be interpreted carefully. Especially kidney function test (BUN and creatinine), electrolytes, and fluid intake should be taken into consideration with attention. RTOG pooled analysis of patients aged 75 or more showed no significant differences in CR or DSS.
4.4 Metastatic Disease and Recurrences
Distant metastasis is much more frequent compared to local control in bladder cancer. In patients with RC, 5-year locoregional control rates are up to 80%, but 5-year OS was ranging from 40 to 60% [16, 46]. Cisplatin and gemcitabine are the treatment of choice in most patients with metastatic bladder carcinoma. Response rates to the first-line treatment are satisfactory, but eventually most of the patients develop progression and need second-line treatment. It is hard to mention cure in metastatic disease, but patients with node-only metastatic disease or lung carcinoma may be cured by chemotherapy. Radiotherapy is always an integral part of treatment in patients with metastatic disease. As in other cancer types, it may be used for palliative intent for local disease such as the bone or brain. Hematuria is the most common cause to refer to radiotherapy in patients with metastatic bladder cancer. Also stereotactic radiosurgery (SRS) has a potential in patients with a small number of metastasis in bladder cancer.
4.5 Irradiation Techniques
Historically, most of the published data about bladder cancer RT is about two-dimensional conventional RT. Nowadays, 3D-conformal RT is the current standard in most of the clinical trials. IMRT has emerged as an option for image guidance. Advances in RT planning, verification, positioning, and delivery provide a means to optimize RT for bladder cancer and overcome difficulties, which have previously limited the success of this treatment, and also offer the opportunity to reduce the irradiated normal tissue volumes while delivering more intensive and increased RT dose.
Intensity modulated radiotherapy (IMRT) is a new technique which allows more accurate delivery of required doses or beyond to the tumor while protecting adjacent organs or structures. This technique is widely used in radiotherapy and replaced the three-dimensional radiotherapy (3D-RT) in the treatment of MIBC. Fractionation schedules and treatment fields vary from centers. There is no randomized trial comparing the conventional fractionation and BID. The most accepted approach is 40–45 Gy to the entire bladder, prostatic/proximal urethra, and the lymph nodes in the pelvis following by boost 20 Gy to the tumor a total dose of 65–66 Gy. Treating only bladder for a total dose of 64 Gy in 2 Gy fractions is performed by some groups (i.e., the UK).
4.5.1 Simulation, Target Volume, and Fields
All simulations are performed with empty bladder in supine position. This makes more reproducible and predictable positioning of bladder. 3D-CT simulation is essential for treatment. Patients underwent 3-mm-slice-thickness CT from mid-L4 to the lower edge of lesser trochanters. Intravenous contrast could be used in suspicious cases, not as clinical routine.
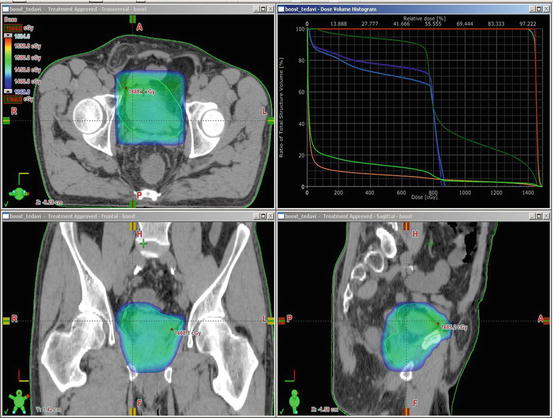
Treatment of urinary bladder cancer involves two phases of RT. The pelvic lymph nodes, prostate, and bladder are target volumes in the first phase. The second phase boosts the bladder alone. The pelvic lymph nodes consist of obturator and internal and external iliac lymphatics. As there might be occult stromal invasion, prostatic urethra and the first 2 cm of proximal urethra need to be covered in men and women, respectively. The top border of the field starts at the level of the bifurcation of the common iliac vessels and does not include the entire pelvis (Figs. 4.2 and 4.3).
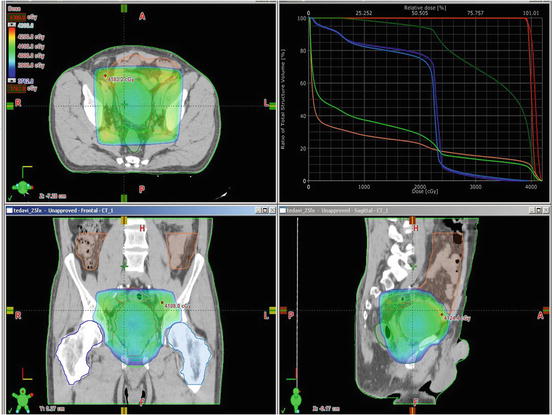
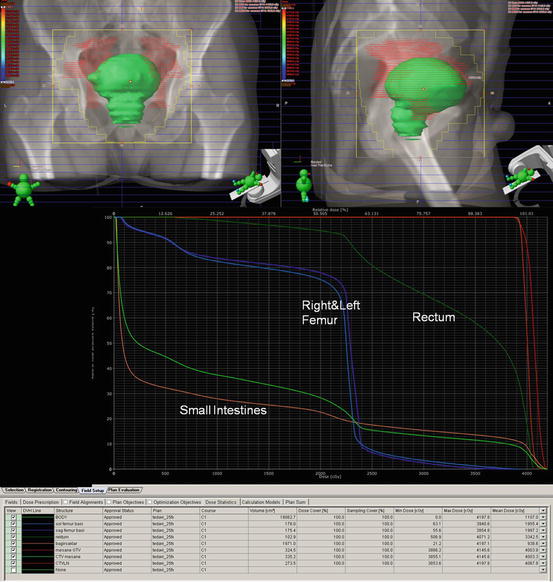

Fig. 4.2
Treatment of urinary bladder cancer involves two phases of RT. The pelvic lymph nodes, prostate, and bladder are target volumes in the first phase

Fig. 4.3
Beam eye views and dose volume histogram for the first phase of radiotherapy
4.5.2 Tumor Localization
Tumor location is essential for determining the target volume. For this purpose, operative notes during cystoscopy and histology reports should be available, and cross-sectional imaging is mandatory. Standard supine position is the treatment position of choice. Computed tomography simulation is the gold standard for treatment planning, for adequate coverage of the whole bladder [47]. Intravesical contrast (30–70 mL) may also be administered via a catheter, with or without additional air (15–30 mL). It is necessary to make sure that the use of contrast does not result in considerable difference of the bladder volume in planning and during daily treatment delivery [48].
Although there is no randomized trial favoring the inclusion of the pelvic nodes in the radiation volume, elective nodal irradiation aims at eradication of micrometastatic disease in the pelvis. However, this approach results in irradiation of a large target volume, encompassing significant amounts of the small bowel and rectum. This can lead to greater gastrointestinal toxicity and potentially limit treatment intensification [49]. The question of whether to include lymph nodes or not in CTV has never been the issue of any randomized trial. Centers that irradiate the bladder alone do not report either increased pelvic failure rates or reduced survival, although direct comparisons are difficult [50]. Retrospective studies comparing patients receiving irradiation to the locoregional lymph nodes with patients treated with small fields to the bladder only showed a significantly worse treatment outcome on irradiation of the nodes [51].
However, the bias of treating the more advanced cases with locoregional nodes may be the reason for these inferior results [52]. However, reviewing the extended pelvic lymphadenectomy data in bladder cancer showed that several prospective and retrospective studies proved that the extent of lymphadenectomy and the number of dissected nodes determine the survival rates, even in node-negative patients [53]. This may reflect the importance of eradication of the disease in the lymph nodes, even in its microscopic form, and in bladder cancer end results. With the advancement in radiotherapeutic techniques, it is expected that radiation can improve the tumor control probability and reduce normal tissue complication probability in such patients.
4.5.3 Target Delineation
The CTV for irradiating the bladder should encompass the entire outer circumference of the bladder, any extravesical disease spread, and any region deemed to be at risk of microscopic disease spread whenever whole bladder irradiation is the plan. For 3D planning, gross tumor volume (GTV) is determined including the bladder with any extravesical extension. Upon irradiation of bladder tumor, the determination of the tumor is mostly guided by MRI-fused images and/or other diagnostic modalities, including cystoscopic bladder maps and fiducial markers placed transurethrally.
Macroscopic visible tumor on CT/MRI or cystoscopy is delineated as gross tumor volume (GTV), if possible. The entire bladder, pelvic lymph nodes (obturator, external and internal iliacs), and prostate and prostatic urethra in men and the 2 cm of the proximal urethra in women are included in the first phase of clinical tumor volume (CTV1). In the second phase of the treatment, pelvic lymph nodes were excluded from clinical tumor volume (CTV2). Small bowel and rectum are delineated at organs at risks (OARs). Individualized planning tumor volume (PTV) margins are used for each center. 1.5–2 cm is the most commonly used isotropic margins for CTV to PTV, and it may be reduced with IGRT. It is shown that bladder wall movement of more than 1.5 cm occurs at least once during a course of treatment in more than 60% of patients (Figs. 4.4 and 4.5) [54].