Although cystectomy remains the standard for treatment of muscle-invasive bladder cancer in the United States, there exist potentially curative alternatives for a selected subset of these patients in organ preservation using concurrent chemotherapy with radiation following an aggressive transurethral resection of the tumor. Chemotherapy and radiotherapy in combination, with salvage cystectomy for invasive recurrence, have produced 10-year disease-specific survival rates of 60% to 65% with overall survival similar to that of cystectomy in selected patients. Fine-tuning of the chemoradiotherapy sequencing, timing, and fractionation has been reported in both single-center and cooperative group publications from North America and Europe.
Key points
- •
Organ preservation by concurrent chemoradiotherapy following an aggressive transurethral resection (TUR) of muscle-invasive bladder cancer (MIBC) is an established treatment of selected patients with bladder cancer as an alternative to cystectomy with or without chemotherapy.
- •
Established patient and disease factors must be considered when deciding to offer patients with bladder cancer organ-preserving chemoradiotherapy, and attention must also be paid to the treatment regimen and the radiation technique.
- •
Ongoing biomarker studies of the TUR of bladder tumor (TURBT) specimen suggest that subsets of patients may respond favorably to an organ-preservation approach using radiation or chemoradiotherapy regimens, but prospective studies are needed to validate these observations.
- •
Concurrent chemoradiotherapy following a complete TURBT for organ preservation is now being studied prospectively for patients with non-MIBC who are refractory to intravesical therapy.
Introduction
Bladder cancer has a significant incidence and mortality, with approximately 75,000 new cases and 15,000 deaths in the United States in 2013. Management of muscle-invasive bladder cancer (MIBC) has evolved significantly over the past few decades. Cystectomy remains the standard treatment, but organ-preservation approaches combining concurrent chemotherapy and radiation following an aggressive transurethral resection of bladder tumor (TURBT) have emerged as appropriate alternatives in selected patients. Although preoperative radiotherapy followed by cystectomy was found to initially improve local control, this approach was later abandoned. Postoperative nonconformal radiotherapy was associated with significant toxicity when earlier radiotherapy techniques were used, but postoperative radiation using intensity-modulated radiotherapy (IMRT) is now again under study. Although the addition of radiotherapy with surgery (in either the preoperative or postoperative study) is not currently standard, the addition of neoadjuvant (and probably adjuvant) chemotherapy with surgery has been demonstrated to improve survival. The combination of surgery and chemotherapy is now the optimal standard in patients suitable for cisplatin-based multidrug therapy from the standpoint of cancer control outcomes, with a 5-year overall survival of approximately 70% for muscle-invasive disease.
The morbidity of surgery must be weighed into consideration, particularly because of the extensiveness of the surgery and associated recovery. In this context, a trimodality therapeutic organ-preservation approach (TURBT plus concurrent chemotherapy and radiation) was developed in order to attempt to achieve a high cancer control rate while preserving good bladder function and a good quality of life. Organ-preservation approaches are now commonly used in other areas of cancer treatment whereby extensive surgeries were once standard: lumpectomy plus radiotherapy in place of modified radical mastectomy for breast cancer, chemoradiotherapy in place of abdominoperineal resection for anal cancer, chemoradiotherapy and limb-sparing surgery in place of amputation for sarcomas, and chemoradiotherapy in place of extensive surgery for head and neck cancers. In all of these disease sites, an organ-preservation technique (with the more extensive surgery reserved for salvage therapy), achieved similar rates of local control and overall survival as up-front extensive surgery.
It is with this same general goal that bladder preservation strategies were developed. Although radiotherapy alone in general has poorer cancer control outcomes in comparison with cystectomy, the addition of chemotherapy to radiotherapy produced results commensurate with cystectomy series. Recently, cooperative group trials (Radiation Therapy Oncology Group [RTOG] 0233 and BC2001) report 5-year cystectomy-free survival rates of more than 60% for selected patients with muscle-invasive disease without any demonstrated compromise to the overall survival in comparison with up-front cystectomy series.
Introduction
Bladder cancer has a significant incidence and mortality, with approximately 75,000 new cases and 15,000 deaths in the United States in 2013. Management of muscle-invasive bladder cancer (MIBC) has evolved significantly over the past few decades. Cystectomy remains the standard treatment, but organ-preservation approaches combining concurrent chemotherapy and radiation following an aggressive transurethral resection of bladder tumor (TURBT) have emerged as appropriate alternatives in selected patients. Although preoperative radiotherapy followed by cystectomy was found to initially improve local control, this approach was later abandoned. Postoperative nonconformal radiotherapy was associated with significant toxicity when earlier radiotherapy techniques were used, but postoperative radiation using intensity-modulated radiotherapy (IMRT) is now again under study. Although the addition of radiotherapy with surgery (in either the preoperative or postoperative study) is not currently standard, the addition of neoadjuvant (and probably adjuvant) chemotherapy with surgery has been demonstrated to improve survival. The combination of surgery and chemotherapy is now the optimal standard in patients suitable for cisplatin-based multidrug therapy from the standpoint of cancer control outcomes, with a 5-year overall survival of approximately 70% for muscle-invasive disease.
The morbidity of surgery must be weighed into consideration, particularly because of the extensiveness of the surgery and associated recovery. In this context, a trimodality therapeutic organ-preservation approach (TURBT plus concurrent chemotherapy and radiation) was developed in order to attempt to achieve a high cancer control rate while preserving good bladder function and a good quality of life. Organ-preservation approaches are now commonly used in other areas of cancer treatment whereby extensive surgeries were once standard: lumpectomy plus radiotherapy in place of modified radical mastectomy for breast cancer, chemoradiotherapy in place of abdominoperineal resection for anal cancer, chemoradiotherapy and limb-sparing surgery in place of amputation for sarcomas, and chemoradiotherapy in place of extensive surgery for head and neck cancers. In all of these disease sites, an organ-preservation technique (with the more extensive surgery reserved for salvage therapy), achieved similar rates of local control and overall survival as up-front extensive surgery.
It is with this same general goal that bladder preservation strategies were developed. Although radiotherapy alone in general has poorer cancer control outcomes in comparison with cystectomy, the addition of chemotherapy to radiotherapy produced results commensurate with cystectomy series. Recently, cooperative group trials (Radiation Therapy Oncology Group [RTOG] 0233 and BC2001) report 5-year cystectomy-free survival rates of more than 60% for selected patients with muscle-invasive disease without any demonstrated compromise to the overall survival in comparison with up-front cystectomy series.
Patient evaluation overview
Risk factors for bladder cancer are smoking, chronic physical irritation (such as bladder stones, schistosoma haematobium , or indwelling catheter), and chronic chemical irritation (such as phenacetin, cyclophosphamide, and occupational factors in the dye, rubber, and paint industries). Urothelial carcinoma (formerly called transitional cell carcinoma) is the most common histologic variant (representing more than 90% of bladder cancer cases in North America), and further discussion in the current article is restricted to urothelial carcinoma. Diagnostic workup initially begins with a thorough physical examination with particular attention to a bimanual examination to determine the extent of pelvic involvement. A cystoscopic/TURBT examination is critical, with biopsies taken from all visualized areas of abnormalities and often random normal areas (including prostatic urethra biopsies, as indicated). The size, number, and morphology of each lesion should be documented as well as a tumor map. It may be important to evaluate by imaging the upper urinary tract for synchronous lesions, although these are rare. The laboratory workup consists of a urinalysis, cytology, and basic chemistry/blood counts. The radiological workup consists of computed tomography (CT) or MRI of the pelvis and abdomen and a chest radiograph or CT; a bone scan is typically obtained for muscle-invasive lesions, particularly if the alkaline phosphatase is elevated. The reader is referred to the American Joint Committee on Cancer’s manual for staging. Discussion in this article is primarily related to muscle-invasive, node-negative, nonmetastatic disease (cT2-T4, cN0, M0) after the aforementioned workup.
Pharmacologic treatment options
A variety of drugs from several different classes have been found to have single-agent activity against metastatic bladder cancer, including (alphabetically) carbogen, cisplatin, doxorubicin, 5-fluorouracil (5FU), gemcitabine, ifosfamide, methotrexate, mitomycin C, nicotinamide, paclitaxel, and vinblastine. Consistent with approaches at other disease sites, several multi-agent regimens have been designed to overcome single-agent resistance as well as to have nonoverlapping toxicity; these regimens have been used in the neoadjuvant, adjuvant, and concomitant settings with surgery and radiotherapy and are summarized in Table 1 .
| Major Drugs with Single-Agent Activity | Neoadjuvant or Adjuvant Regimens (with Surgery or Radiotherapy) | Concomitant Agents/Regimens with Radiotherapy |
|---|---|---|
| Carbogen Cisplatin Doxorubicin 5FU Gemcitabine Ifosfamide Methotrexate Mitomycin C Nicotinamide Paclitaxel Vinblastine | MVAC MCV (also called CMV) ITF Gemcitabine & cisplatin Gemcitabine & paclitaxel | Cisplatin 5FU & cisplatin Paclitaxel & cisplatin Gemcitabine (low dose) 5FU & mitomycin C Carbogen & nicotinamide |
Nonpharmacologic treatment options
Surgical Treatment Options
A range of surgical options is available, ranging from minor office procedures to major operating room procedures requiring weeks of recovery time. The TURBT is the standard of care for non-MIBC (N-MIBC) and typically serves a diagnostic role for MIBC. A partial cystectomy, which involves extraperitoneal cystostomy and wide local excision, is indicated for a solitary, well-defined tumor with no evidence of carcinoma in situ in a mobile portion of the bladder and for which no ureteral implant is needed; only approximately 5% of patients with muscle-invasive disease are candidates for partial cystectomy. The next most aggressive procedure is the simple cystectomy, in which the plane of dissection is the bladder wall; this procedure involves the removal of the bladder plus various amounts of ureters/urethra and is a palliative procedure only; the standard curative surgery for muscle-invasive disease is the radical cystectomy.
In men, radical cystectomy involves the removal of the bladder, prostate, seminal vesicles, proximal vas deferens, distal ureters, proximal urethra, and the margin of the adipose and peritoneum and includes a pelvic lymph-node dissection. In women, the procedure involves the removal of the bladder, urethra, uterus, oviducts, ovaries, anterior vaginal wall, distal ureters, and the surrounding adipose tissue/fascia and also involves a pelvic lymph-node dissection; note that in women, the radical cystectomy is synonymous with an anterior exenteration. Urinary diversions fall into 2 major categories: incontinent and continent. Incontinent diversions, in which there is no internal reservoir, are typically conduit diversions, the most common of which is the ileal conduit. Continent diversions typically involve diverting the ureters into a resected segment of ileum or ileocecum that is then either fixed to the abdominal wall with an external stoma created or anastomosed to the urethra in men or the sigmoid colon in women. The continent diversion can either require self-catheterization or can function similar to that of a normal bladder.
Radiotherapy Treatment Options
External beam radiotherapy (EBRT) alone, as described earlier, typically has lower cancer control rates than radical cystectomy for a given stage; its use as a sole treatment is typically reserved for those who are poor surgical candidates, poor organ-preservation chemoradiotherapy candidates, have metastatic disease with a symptomatic bladder issues, or have unresectable locally advanced disease. Interstitial brachytherapy can be used for selected patients either alone or in combination with EBRT, both in the context of primary treatment with radiotherapy or as adjuvant treatment in conjunction with partial cystectomy. Further discussion herein is restricted to EBRT as the radiotherapy modality.
Combination therapies
Surgery Plus Radiotherapy
Preoperative or neoadjuvant irradiation was explored in several randomized trials, with several different fractionation schemes evaluated. Although a local control benefit was demonstrated and a dose response was suggested in several studies, the practice has largely been abandoned.
Postoperative/adjuvant radiotherapy was investigated in an RTOG/Jefferson study (conducted during an era of older treatment techniques) with toxicity found to be prohibitively high. Postoperative radiotherapy remains an active area of investigation (in the context of modern treatment techniques) with several ongoing single-institution phase II studies as well as a randomized phase II protocol being developed by the NRG cooperative group/National Cancer Institute clinical trial network in patients with pT3/4 and/or pN+ disease.
Surgery Plus Chemotherapy
Neoadjuvant chemotherapy with surgery has several theoretic advantages: early therapy for potential micrometastases, drug delivery not being compromised by an altered surgical bed, and improved tumor resectability. Neoadjuvant chemotherapy has been found in randomized trials to improve disease-free survival and overall survival over surgery alone.
Adjuvant chemotherapy also has several theoretic advantages: not delaying the definitive local therapy and knowledge of pathologic staging (and consequent application for adverse disease features, such as positive nodal status, extravesical tumor extension, and/or lymphovascular invasion). With respect to adjuvant chemotherapy, although earlier meta-analyses did not suggest a benefit to this strategy, a more recent meta-analysis suggests a disease-free survival and overall survival advantage with adjuvant cisplatin-based chemotherapy after surgery; this remains an area of active investigation.
Chemotherapy Plus Radiotherapy
Chemotherapy in combination with radiotherapy has 2 goals. First, as the case when combined with surgery, one goal is to eradicate distant micrometastases and to improve distant control. The other goal is radiosensitization. The overall objective is to achieve local control and distant control while achieving organ preservation and, thus, reserving an extensive surgical procedure for salvage therapy.
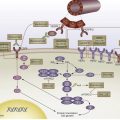
The general flow path as practiced in the US cooperative group setting consists of (1) complete TURBT; (2) induction chemoradiotherapy; (3) response assessment with cystoscopy/biopsy; (4) if a complete response is achieved, consolidation chemoradiotherapy; (5) further response assessment with cystoscopy/biopsy; and (6) continued surveillance. The aforementioned treatment describes a split-course regimen; one alternative is to omit the midtreatment course response assessment (item 3 previously mentioned) and deliver the chemoradiotherapy in a single course. With either regimen (full course or split course), salvage cystectomy is curative for many poorly responding patients or those who recur with an invasive tumor. Fig. 1 shows the aforementioned flow path; note that this flow path does not show a step for neoadjuvant chemotherapy that had been used in some earlier regimens (and may warrant further investigation).