T – primary tumor
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Ta
Noninvasive papillary carcinoma
Tis
Carcinoma in situ: “flat tumor”
T1
Tumor invades subepithelial connective tissue
T2
Tumor invades muscle
T2a
Tumor invades superficial muscle (inner half)
T2b
Tumor invades deep muscle (outer half)
T3
Tumor invades perivesical tissue
T3a
Microscopic
T3b
Macroscopic (extravesical mass)
T4
Tumor invades any of the following: prostate, uterus, vagina, pelvic wall, abdominal wall
T4a
Tumor invades prostate, uterus, or vagina
T4b
Tumor invades pelvic wall or abdominal wall
N – lymph nodes
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Metastasis in a single lymph node in the true pelvis (hypogastric, obturator, external iliac, or presacral)
N2
Metastasis in multiple lymph nodes in the true pelvis (hypogastric, obturator, external iliac, or presacral)
N3
Metastasis in common iliac lymph node(s)
M – distant metastasis
M0
No distant metastasis
M1
Distant metastasis
16.2 General Treatment Concepts and Indications for Radiotherapy
16.2.1 Treatment Concepts and Therapy Results in Superficial, Non-Muscle-Invasive Bladder Cancer (NMIBC, Ta, Tis, T1)
Over 80 % of newly diagnosed cases are classified as Ta, Tis, or T1. TUR (transurethral resection) and (in case of risk factors) additional intravesical cytostatic therapy, mainly with bacillus Calmette-Guérin (BCG), are the important cornerstones of initial treatment. The recurrence and progression rate of NMIBC is strongly associated with tumor grade and invasion into the lamina propria. Early cystectomy for high-risk superficial cancers has been recommended because of favorable results in some series, but it is not clear whether the results reflect the efficacy of surgical therapy or are only caused by patient selection. However, patients with failure after TUR and intravesical therapy should be considered for more intense treatment with the decision based on T-category, tumor multiplicity, size, concomitant in situ cancer, and urothelial tumor of the prostatic urethra. Cystectomy is considered as the standard treatment for this subgroup, but concurrent chemoradiotherapy might be an alternative because of good results in some series (Weiss et al. 2006).
16.2.2 Treatment Concepts and Therapy Results in Localized Muscle-Invasive Bladder Cancer (T2–T4 N0/N1 M0)
For localized muscle-invasive bladder cancer, radical cystectomy has been widely considered as the standard treatment. One ancient randomized study showed a nonsignificant advantage of cystectomy over radiotherapy (radiation alone without chemotherapy). However, numerous phase II studies with concurrent chemoradiotherapy have demonstrated survival rates identical to surgical series with 70–80 % bladder preservation in long-term survivors. The most impressive advantage with regard to survival has recently been shown in the British BC 2001 study which demonstrated the superiority of concurrent chemoradiation over radiotherapy alone. The absolute difference in survival was 13 % after 5 years which is higher than the increase in survival with adjuvant or neoadjuvant chemotherapy prior or after radical cystectomy. Thus, chemoradiation is the standard of care for organ preservation and is, with regard to survival, to be considered as equieffective to cystectomy according to the best available evidence at the moment (Fig. 16.1).
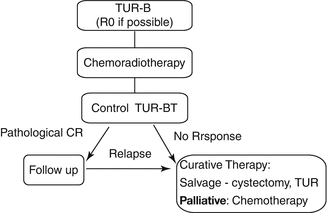

Fig. 16.1
General treatment concept for an organ-preserving treatment approach
16.2.3 Indications for Definitive Curative Radiotherapy
Radiotherapy is an attractive curative option for nearly all patients with muscle-invasive bladder cancer with the additional advantage of bladder preservation in the majority of patients. Standard treatment for patients with curative approach is radiotherapy plus concurrent chemotherapy. The best chemotherapy regimen is not yet clear. The most widely used drug has been cisplatin (Rodel et al. 2002). However, a combination of 5-FU and mitomycin C has also demonstrated significant efficacy in the BC 2001 trial and is surely an evidence-based alternative to cisplatin (James et al. 2012). A further drug for radiosensitization is paclitaxel which has been shown to be safe and efficacious in patients with contraindications to cisplatin (Müller et al. 2007).
All patients should initially undergo a complete transurethral resection of the bladder tumor (TUR-BT). The TUR should be complete, if possible; a macroscopically complete TUR-BT is a major prognostic factor. TUR-BT is followed by chemoradiotherapy. About 6 weeks after chemoradiotherapy, a control cystoscopy is recommended. A pathologically complete remission (pCR) can be achieved in about 70 % of all patients and a pCR is a prognostic factor for survival, long-term tumor control in the bladder, and bladder preservation (Jenkins et al. 1988; Mameghan et al. 1995). Patients with residual invasive tumor on control cystoscopy should undergo salvage cystectomy, if there are no contraindications to major surgery.
Start of radiotherapy is recommended 4–6 weeks after TUR-BT because some time is normally required until local symptoms after TUR (dysuria, urgency) have been solved.
16.2.4 Adjuvant Radiotherapy
The role of adjuvant radiotherapy is not well defined with very limited data. Preoperative radiotherapy has been used in historical studies but is not considered as standard in contemporary guidelines. Preoperative chemoradiation followed by cystectomy is, from a theoretical point of view, an attractive concept but should only be used in clinical trials.
16.2.5 Palliative Radiotherapy
Palliative radiotherapy is useful for the relief of symptoms such as bleeding and reduces the pain for patients with T4 bladder tumors, pelvic nodal disease, and bone and other distant metastases. Short courses of palliative pelvic radiotherapy may be beneficial for elderly patients who have significant comorbidities precluding radical treatment.
16.3 Normal Anatomy and Typical Tumor Spread
16.3.1 Anatomy of the Bladder
The bladder is located on the floor of the pelvic cavity. It is anterior to the rectum, seminal vesicles, and ureters in males. In females it is anterior to the uterus and upper vagina. As the bladder distends, the neck remains fixed and the dome rises into the pelvic cavity. The bladder is a musculomembranous sac. It appears smooth when full and has numerous folds when it is empty.
16.3.2 Spread of Primary Tumor
Tumors may grow papillary or as solid-infiltrative lesions. In papillary tumors, most of the tumor volume extends into the bladder. The majority of the advanced lesions, however, grow as flat lesions (so-called solid-infiltrative lesions) with most of the tumor in the bladder wall or beyond.
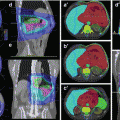
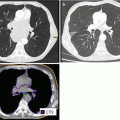
It is obvious from surgical series that all diagnostic modalities bear a large risk of staging error. It is nearly impossible to clearly differentiate between T1 (no muscle invasion), T2a (superficial muscle invasion) and T2b (deep muscle invasion), and extravesical extension (T3). Even if patients are staged by cystoscopy, TUR, and the best available diagnostic modalities (CT, MRI), a significant staging error with regard to T-category remains. In a recent large multicenter trial, even the use of nomograms prior to radical cystectomy underestimated the risk of locally advanced disease in nearly half of all patients. In large surgical series, extravesical disease (T3–T4) is present in more than two-thirds of all patients with proven muscle invasion. Therefore, it is justified and useful to use standard target volumes for the majority of patients and to adapt the standard volumes in cases with large tumor masses.
16.3.3 Spread to Regional Lymph Nodes
In surgical series, about one-third of patients treated with cystectomy for muscle-invasive cancers have nodal involvement (Yafi et al. 2011). Surgical series and sentinel-node series indicate there is no clear step-by-step involvement of regional nodes as, for example, known from axillary involvement in breast cancer. A single involved node may lie more or less randomly in the regional node areas (Leissner et al. 2004; Bruins et al. 2014). However, early nodal involvement is often localized in the paravesical and obturator nodes in direct neighborhood to the bladder (laterally between the bladder and iliac external vessels and behind the bladder). Thus, radical treatment of lymph nodes requires dissection or irradiation of all regional nodes irrespective of tumor volume and stage. On the other hand, a limited nodal treatment with treatment only to the obturator nodes and internal iliac nodes would cover all involved nodes in the majority of node-positive patients.
The optimal extent of lymph node treatment is not well defined. Data from retrospective surgical series suggest that an extended nodal resection might be superior in terms of survival (e.g., Skinner 1982), but this hypothesis is highly controversial in the light of negative results of extended lymph node dissection in all other cancer types where such procedures have failed to demonstrate a survival benefit despite positive data in retrospective series, e.g., in gastric cancer or breast cancer. Moreover, a recent study from a single institution compared different target volumes (whole pelvis versus bladder only) in patients undergoing definitive radiotherapy and found no difference in survival (Tunio et al. 2012).
16.4 Target Volume
16.4.1 Evidence-Based Recommendations for Target Volume of the Primary Tumor
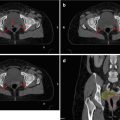
Most of the treatment series have used whole-bladder radiotherapy, either for the complete treatment course or combined with a partial-bladder boost. Arguments in favor of whole-bladder irradiation are the difficulties to clearly define tumor borders in the bladder wall with imaging methods, the risk of multiplicity of tumors in the bladder, and the changes in bladder filling during treatment with subsequent difficulties to exactly locate the tumor. On the other hand, partial-bladder irradiation offers the advantage to reduce acute bladder toxicity and to increase the total dose to the macroscopic tumor. Recently, two randomized studies on this issue have been published. In the BC 2001 study, a subgroup of patients was randomized between whole- and partial-bladder irradiation with no obvious differences in outcome. A second randomized study from Manchester compared whole-bladder irradiation with a standard dose (52.5 Gy in 20 fractions) to dose-escalated partial-bladder irradiation (primary tumor plus 1.5 cm margin, 57.5 Gy in 20 fractions or 55 Gy in 16 fractions) in patients with unifocal T2–T3 N0 M0 cancers and found also no significant difference (Cowan et al. 2004). This second study suggests that, firstly, partial-bladder irradiation with modern imaging and treatment techniques (3D-CRT) does not increase failure rates and, secondly, that the moderate increase in dose did not increase local control or survival. However, the long-term bladder preservation rate in the Manchester study was, in absolute figures, slightly better with whole-bladder as compared to partial-bladder irradiation (Table 16.2), and the patient numbers in both arms were so small that a clinically relevant difference with regard to bladder preservation might not have been detected. Moreover, there was a trend toward increased bladder toxicity in the dose-escalated arms, especially in patients treated with 16 fractions in 3 weeks (Table 16.2).
Table 16.2
Randomized study of whole-bladder irradiation with standard doses versus partial-bladder irradiation with escalated dose in patients with unifocal cT2–T3 N0 bladder cancer
Whole bladder | Partial bladder | ||
|---|---|---|---|
Standard dose | Dose escalated | ||
N
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||